How "bodies on a chip" can transform animal welfare
 Getty Images
Getty Images“Bodies on a chip” serve as proxies for living beings, alleviating the need for lab animals, inspiring new cures for rare diseases and even offering a way to resuscitate dying species
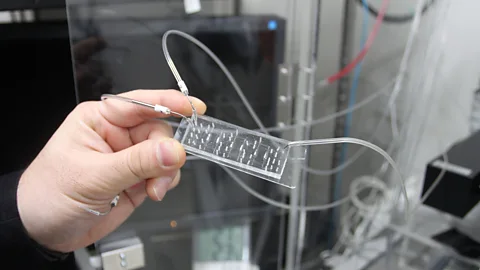
When Ken-Ichiro Kamei, a microengineer at Kyoto University, goes out drinking with his friends, he usually brings along one of his “bodies on a chip.” When the topic of work inevitably comes up, he’ll whip out the chip – which looks like a lab slide, but with an added crystal-clear silicone rubber layer containing faintly visible troughs and channels – and declare, “I’m making these devices to recreate humans and animals.”
Wows inevitably ensue. “It’s like I’m a magician and my friends have asked me to do some tricks,” Kamei chuckles.
Kamei is at the forefront of a new field of biotechnology that seeks to replicate organs, systems and entire bodies on chips such as the one he likes to show off. While traditional biochemical experiments carried out on lab plates are static and isolated, the chips Kamei uses contain an interconnected system of channels, valves and pumps that allow for more complex interactions – to the point that they can mimic a living system. Recognizing the potential such chips have for revolutionizing medical research, in 2016 the World Economic Forum named “organs-on-chips” in their top 10 emerging technologies of the year. But while those specialised chips mimic particular tissues or organs, Kamei and his colleagues aim to eventually mimic whole animals. “It’s quite ambitious,” he says.
 Rachel Nuwer
Rachel NuwerKamei builds his own microfluidic chips in his lab, primarily using a laser cutter and a 3D printer. To operate the chips, he adds various types of cell tissue into six chambers connected to microchannels and then hooks the chip’s pneumatic micropumps up to a controller to create circulation. This gives him and others the ability to test the efficacy and side effects of new drugs, to design personalised medicine for individuals based on their cell cultures and to better understand the underpinnings of disease. In one experiment, for example, Kamei and his colleagues loaded a chip with healthy heart cells and cancerous liver cells. They then added doxorubicin, an anti-cancer drug known to cause toxic side effects on the heart but whose specific mechanism to toxicity was unknown. The researchers discovered that the drug did not directly cause the heart damage; instead, the metabolised by-product produced by the liver did.
Such experiments require an ample supply of diverse cells, and would not have been possible were it not for the work of Shinya Yamanaka, a stem cell researcher at Kyoto University who won the 2012 Nobel Prize in Physiology or Medicine for his pioneering creation of induced-pluripotent stem (iPS) cells. “iPS cells can proliferate many, many times outside of the body, whereas other types of stem cells cannot,” Kamei says. “Previously-used cell lines also came from just one person, which wasn’t useful for studying genomic diseases or a specific individual.”
As their name implies, undifferentiated iPS cells can be induced from virtually any other type of cells in the body. The cells are transformed using “Yamanaka factors,” or protein-coding genes that reprogram cells into an embryonic state. The blank canvas cells can then be coaxed into becoming any other type of cells, including sperm cells and eggs for fertility treatments, or any others in the body for pharmaceutical trials.
 Rachel Nuwer
Rachel NuwerLike most biomedical technologies, iPS cells and chips such as the one Kamei uses were created with humans in mind, not animals. But both technologies have great potential for aiding in species conservation as well as animal welfare. iPS cells could be used to synthesise lab-grown meat, for example, alleviating inhumane treatment of livestock and environmentally harmful side effects from farming, or to create endangered species products that satisfy market demand without killing wildlife. As in humans, chips also open up a vehicle for studying and better understanding wildlife – and therefore better protecting it.
If you like this, you may also enjoy:
“Many scientists are excited about the possibilities for these kinds of technologies to benefit a broader context than human medical applications,” says Oliver Ryder, director of conservation genetics at the San Diego Zoo Institute for Conservation Research and a partner on Kamei’s “body on a chip” project. “It’s very fortunate that, within the context of shared interests, this research program could play an important role in animal conservation.”
 Getty Images
Getty ImagesIt was animal welfare, not conservation, that originally prompted Kamei to look beyond the confines of human medicine. While studying laboratory mice at the University of California, Los Angeles, he found himself sympathizing with his four-legged subjects. “I was like, why do I need to use a mouse to study humans?” he recalls. “I was curious about how I could help such animals.”
He’s not alone in asking that question. Animal testing is going out of vogue in industries and universities around the world. In 2009 the EU banned the practice in its cosmetics industry, and in 2013 lawmakers bumped up protections to include all cosmetics sold throughout the EU, regardless of where they were made. Chips loaded with human tissues can help alleviate the need for animal testing – a double plus since mice, rats, rabbits and monkeys do not always react to a drug or product in the same way people do. Because of this, the human body-mimicking chips, Kamei says, are “considered a main contender for alternatives to animal tests.”
Humans, of course, aren’t the only species that suffer from disease, and iPS cells and chip technologies might accelerate the development of new medical treatments for animals too. Significantly fewer people study animal diseases compared to human ones, and fewer resources are available to support those studies. The versatility of wildlife makes it even more difficult to devise species-specific cures for diseases, and on top of that, endangered species tend to be scarce and laws often prohibit capturing them, even if it would help scientists to understand their health and illnesses.
 Getty Images
Getty Images“If we can create organs of endangered animals, we can understand how those organs work and how to protect them from infection,” says Miho Murayama, director of the Wildlife Research Center at Kyoto University. “That would be very useful, because we can’t experiment on them like we do mice.”
Scientists working in Kazakhstan are still struggling to understand why 200,000 saiga antelopes – 60% of the global population – suddenly dropped dead from a bacterial infection in 2015, while researchers in Tasmania have been working for years to devise life-saving treatments for a gruesome, contagious face cancer that is threatening the survival of Tasmanian devils. Gorillas are another prime example: they are notoriously prone to heart attacks – but no one knows why, and no one has been able to provide a fix. “If we can mimic gorilla heart attacks inside the body on a chip system, we can identify what kinds of drug and treatments will help them,” Kamei says. “This kind of testing would be beneficial not only for endangered animals, but also for pets and livestock.”
Ryder adds that beyond chips, iPS cells open up a seemingly endless array of possibilities for species conservation. “If genetic diversity can be banked and restored by turning cells into animals or by using cellularly-based technologies to restore genetic variation, then there’s less risk of extinction,” he says. “It’s amazing to be at a place where we can investigate the possibilities of this kind of technology.”
Ryder is one of the leads on the most well-known of such projects: an international effort to save the northern white rhino – a subspecies of white rhino that is now reduced to just two living individuals – by using frozen tissue samples of former individuals to create iPS cells. The iPS cells would, in turn, be made into egg and sperm cells to create viable, genetically diverse embryos to be implanted in surrogate southern white rhino mothers. While an ambitious start, Ryder points out that this is now the only hope for saving the subspecies from extinction. And regardless of the project’s success, it will likely pave the way for similar species-saving efforts in the future.
 Getty Images
Getty ImagesTechnology, Murayama adds, can also accelerate our understanding of fundamental biology and evolution. She and her colleagues are interested, for example, in how hormones and neurotransmitters like serotonin affect animal behaviour and how gene function underlies those behaviours. To do this, she often relies on genome analysis of individual animals, but to understand real-time function and physiology, nothing beats side-by-side comparisons of living cells. Chips and iPS cells made from some of the 600 species whose genetic information she has collected over the years will better allow her to compare the underpinning of behaviour across species.
“We still don’t have basic information about many wild animals,” Murayama says. “Our goal is to connect field data and lab data, to better understand species.”
Challenges for realizing such goals abound, however. For one, the formula for creating iPS cells differs from species to species. What works for a rhino will not necessarily work for a chimpanzee or an eagle. After iPS cells are made, the differentiation process to grow various cell types can also vary by species, as can the culture conditions necessary for the cells to proliferate and thrive.
Hitomi Tabata and Tomoka Hirayama, students at Hiroo Gakuen High School in Tokyo, recently discovered this first-hand when they tried to create iPS cells from elephants. Like naked mole rats, elephants are exceptional in that they hardly ever get cancer. Tabata and Hirayama – both of whom plan to become doctors with an emphasis on research – chose to study elephants because of the medical potential of their anti-cancer abilities. But in the course of their research they learned about the poaching crisis in Africa, which has cost the lives of tens of thousands of elephants across the continent over the past decade. The students realised that their health-focused project could also yield a conservation solution: lab-grown ivory made from elephant iPS cells.
“We were thinking, if differentiation into ivory is successful, it could help to increase elephant populations,” Tabata says. “We could grow ivory in blocks that would be easy to manufacture into hankos” – the name seals that account for 80% of ivory use in Japan.
 Getty Images
Getty ImagesTabata and Hirayama were able to create pluripotent stem cells from mice, but when it came time to do so with elephants, they ran into a problem. Elephants’ many copies of p53, the gene responsible for the species’ resistance to cancer for the role it plays in resisting the cellular cycle, also made elephants’ cells resistant to reprogramming. “This gene works to resist or reduce the cellular cycle, which means it’s more difficult to create iPS cells,” Hirayama says. Still, she and Tabata plan to keep trying by attempting to knockdown p53 and by changing how they introduce the Yamanaka factor – at least until their graduation in March, after which they hope junior students will take up the project.
Kamei agrees that p53 is a major stumbling block – but one that, like all challenges in science, is worth trying to overcome. “I’m not saying it’s impossible, but it’s almost impossible to reprogram elephant cells because of p53,” he says. “If it is possible, though, I want to do it.” The project does, at least, show how the "bodies on a chip" can provide a useful tool to introduce young scientists to genetics research.
For now, Kamei has created a “body on a chip” mouse model, and he is nearing completion of another for Grevy’s zebras, whose cells arrived at his lab courtesy Murayama’s connections at the Kyoto City Zoo. Dolphins and horses are next on the list. “Each species has hurdles, but they also all present interesting topics to study,” he says. “If my research can be helpful for people working in zoos or with animals, then that’s great.”
He adds, though, that his goals extend even higher – literally beyond the bounds of earthly problems, all the way into space. In the US, the National Center for Advancing Translational Science and the International Space Station US National Laboratory already created a “Tissue Chips in Space” project to test the effects of space on human cells and organs, and Kamei believes they could be of equal value in ensuring animals can make a smooth transition to a post-Earth future. “Humans won’t be the only ones going into space – pets and livestock will, too,” Kamei says. “While I won’t be going to Mars, it’s my dream to help those who do.”
--
Rachel Nuwer is an award-winning journalist who reports about science, travel, food and adventure for the New York Times, National Geographic, BBC Future and more. She is the author of Poached: Inside the Dark World of Wildlife Trafficking.
If you liked this story, sign up for the weekly bbc.com features newsletter, called “If You Only Read 6 Things This Week”. A handpicked selection of stories from BBC Future, Culture, Capital, and Travel, delivered to your inbox every Friday.
Japan: Untold Stories
Welcome to BBC Future Now's Japan season, in which we explore the country's most exciting medical, technological, environmental and social trends.
You can discover:
Can a high-carb diet explain why Okinawans live so long?
Artificial meteors: the world's most ambitious fireworks?
The grand plans to mine ocean vents
The samurai swordsmith designing a spaceprobe
Why 'flammable ice' may solve an energy crisis
Ikumen: The rise of Japan's 'hunky dads'
The 'cathedral' protecting Tokyo from floods

