Coronavirus vaccines: The plan for Northern Ireland
 Getty Images
Getty ImagesNorthern Ireland's biggest ever vaccination programme is being rolled out weeks ahead of schedule, Health Minister Robin Swann has said.
The first vaccine was approved for UK use in early December and within days it was being given to vaccinators in NI, as well care home residents and staff.
Since then, two more vaccines have also been approved by UK regulators.
People in groups most at risk of dying or becoming seriously ill as a result of Covid are being given priority.
Which vaccines are being used?
Three vaccines have been approved for use in the UK:
- the Pfizer-BioNTech vaccine
- the Oxford-AstraZeneca vaccine
- the Moderna vaccine
The first two are already in use in Northern Ireland, but supplies of the Moderna vaccine are not expected to arrive in the UK until the spring.
Each of the approved vaccines is given to patients in two doses, a number of weeks apart.
When will I be vaccinated?
Patients are being offered vaccines in order of priority, based on age and clinical vulnerability, as Covid-19 poses most danger to people who are elderly or who have underlying health problems.
On 12 January, Northern Ireland's Department of Health published a detailed timetable for delivering vaccinations to prioritised groups:


On Friday February 19, the department announced that carers over the age of 18 could also now book their vaccine appointment.
Prioritisation was decided by the Joint Committee on Vaccination and Immunisation (JCVI), which advises UK health departments on immunisation.
It is expected that mass vaccination will only become available to the general population from the summer of 2021.
How many vaccinations have been carried out in Northern Ireland?
The latest available figures from the Department of Health show a total of 418,209 vaccines had been administered by 17 February.
Of that figure, 29,476 jabs were second doses.
As of 16 February the following had received first doses:
- 94% of over-80s
- 88% of 75-79
- 75% of 70-74
- 62% of 65-69
- 23% of people who are clinically extremely vulnerable
How many care home residents have been vaccinated?
By 4 February every one of Northern Ireland's 483 care homes had been visited by mobile vaccination teams, while 90% of homes had been visited for second doses.
Earlier, the Department of Health said there had been an uptake level of more than 90% among residents - and about 80% among staff.
When did vaccinations begin?
Six days later, Belfast nurse Joanna Sloan became the first person in Northern Ireland, and indeed on the island of Ireland, to be vaccinated.
The 28-year-old leads the Belfast Health Trust's vaccination programme.
Later on 8 December, the first care home residents in Northern Ireland were vaccinated at Palmerston residential home in east Belfast.
The Oxford-AstraZeneca vaccine was approved for use in the UK on 30 December 2020.
On 4 January 2021, 94-year-old Eileen Lynch became Northern Ireland's first recipient.
The pensioner said she felt "delighted and privileged" after getting the injection at her GP surgery on the Falls Road in west Belfast.
The UK regulator, the Medicines and Healthcare products Regulatory Agency (MHRA), confirmed the US-made Moderna vaccine was safe and effective to use on 8 January.
The UK has pre-ordered 17m doses, but supplies are not expected to arrive until spring 2021.
Is one vaccine better than the others?
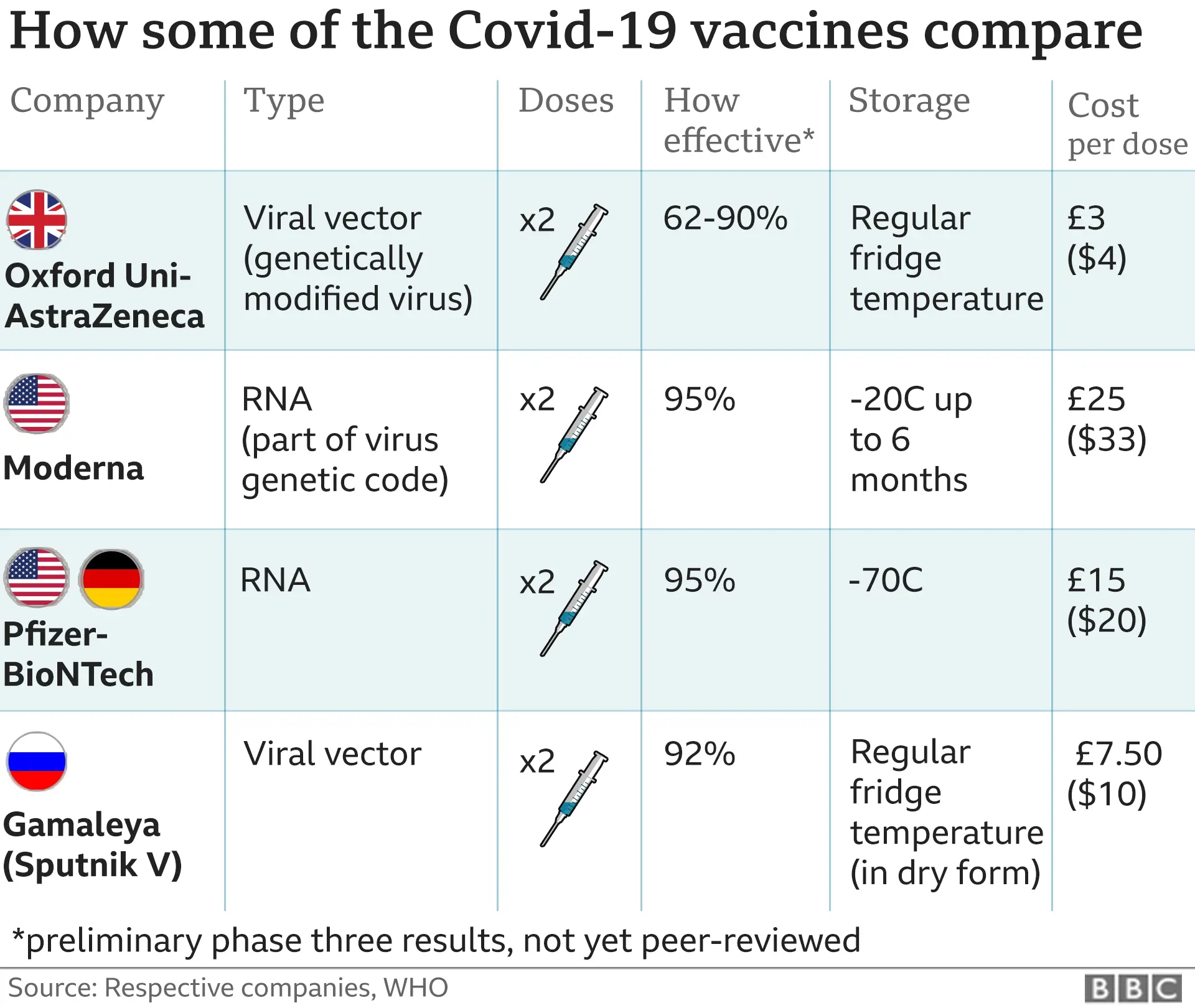
Pfizer-BioNTech, which produced its vaccine in Belgium, has reported 95% protection against Covid-19.
The Moderna vaccine offers similar protection at nearly 95%, while trials of the Oxford-AstraZeneca vaccine showed it was between 62% and 90% effective.
Research from Oxford University found the AstraZeneca vaccine offered 76% effective protection from a single dose for three months.
The Pfizer-BioNTech product is an RNA vaccine that uses a tiny fragment of genetic code from the pandemic virus to teach the body how to fight Covid-19 and build immunity.
An RNA vaccine had never been approved for use in humans before, although people have received such a vaccine in clinical trials.


The Moderna vaccine uses the same RNA approach as the Pfizer vaccine and reported similar effectiveness.
The Oxford-AstraZeneca vaccine is made from a weakened version of a common cold virus (known as an adenovirus) from chimpanzees.
It has been modified to look more like the coronavirus, but cannot cause illness.
The jab prompts the immune system to start making antibodies and primes it to attack any coronavirus infection.
Why do I need two doses?
All the approved vaccines require two doses to provide the best possible protection.
Initially, the strategy for the Pfizer vaccine was to offer people the second dose 21 days after their initial jab - full immunity starts seven days after the second dose.
But when approval was announced for the Oxford-AstraZeneca vaccine on 30 December, it was also announced that the new priority would be to give as many people a good level of protection with the first shot of either vaccine, rather than providing the required two doses to a smaller number of people in as short a time as possible.
Everyone will still receive their second dose, but this will now be within 12 weeks of their first.
The US regulator and some UK experts have questioned the policy, saying it is premature without more trial evidence, but the UK's MHRA says it is a pragmatic decision that will protect more people.
An Oxford University study has suggested that, with no fall in protection during the three-month period following the first jab, research supported gaps between first and second doses of between four and 12 weeks.
Can different vaccines be mixed and matched?
The official guidance states that every person should get the same vaccine for both doses.
However, in the very rare circumstance in which only one vaccine is available at a vaccination site or it's unknown which product an individual received for their first dose, Public Health England has advised a different vaccine could be administered.
But this advice does stress "this option is preferred if the individual is likely to be at immediate high risk or is considered unlikely to attend again".
Which vaccine will I get?
Experts have concluded that both vaccines are very effective, and have not stipulated a preference for either one in any specific population.
In Northern Ireland, care home residents were given the Pfizer jab, while other people aged 80 and over were called to their GP surgery to receive the Oxford-AstraZeneca vaccine. Housebound patients have also received this vaccine.
In January, the Department of Health announced a new twin-track approach for younger groups.
This allowed people aged 65-69 to book slots at their local vaccination centres, where they received the Pfizer jab.
People aged over 70 were to attend their GP and receive the Oxford-AstraZeneca jab.
However on 17 February it was announced that any over-70s who had not yet been contacted by their GP to come for vaccination could book a slot for the Pfizer vaccine at a local centre.
The Pfizer jab at the vaccine centres is also being given to people in NI who received a shielding letter as they are clinically extremely vulnerable (CEV) as well as carers who are in receipt of caring benefits, are registered care home partners or whose illness would jeopardise the welfare of the person they care for.
Meanwhile people aged 18-64 with underlying health conditions - as well as their carers - will be contacted by GPs for an Oxford-AstraZeneca jab.
There are some questions over when exactly people with asthma will get vaccinated as some will be in the 18-64 group with underlying conditions, some in the CEV group and some will not fall into any group.
Their GP will assess the severity of their condition and they will then be assigned to a group for vaccination priority.
Will everyone be vaccinated?
The eventual aim is that as many people as possible over the age of 18 receive a Covid-19 vaccine.
It won't be compulsory, though - no other vaccines in the UK are - as experts say this wouldn't help create confidence in the vaccine.
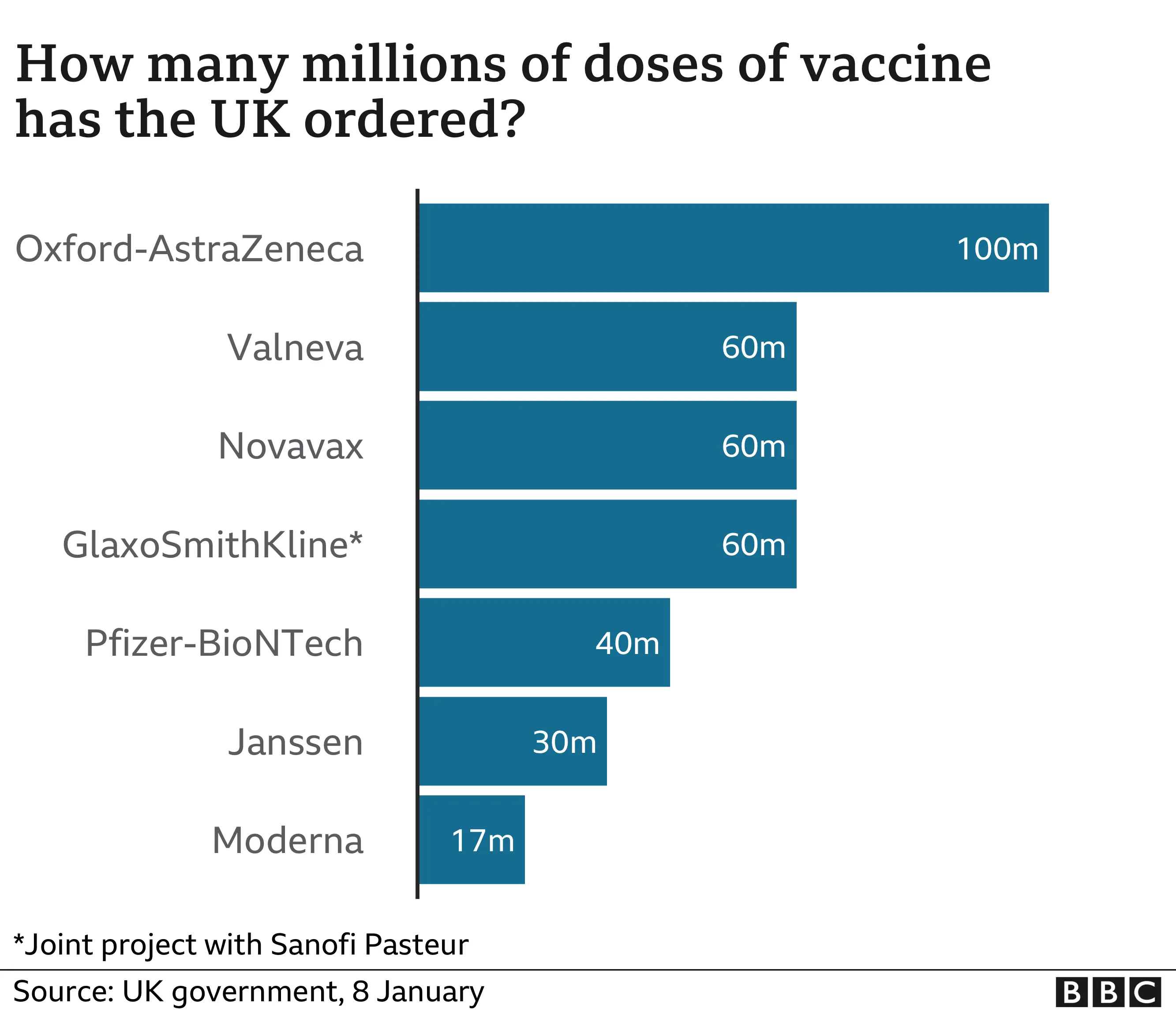
The government has so far ordered seven different types of vaccine and expects to receive 367 million doses.
If everyone needs two doses, that would certainly be enough for every adult in the UK.
What about people with allergies?
Anyone with a previous history of allergic reactions to the ingredients of the vaccine should not receive it, but those with any other allergies such as a food allergy can now have the vaccine.
A severe allergic reaction - known as anaphylaxis - is a very rare side-effect with any vaccine, but it can happen in those at risk. Most people, however, will not be affected in any way.
The medical regulator, the MHRA, says anyone due to receive their vaccine should discuss any medical history of serious allergies with their healthcare professional beforehand.
I'm pregnant - will that affect when I'm vaccinated?
Vaccination with either vaccine should only be considered for pregnant women when the potential benefits outweigh any potential risks - for instance where the risk of exposure to coronavirus is high and cannot be avoided, or where the woman has underlying health conditions that put her at high risk of complications of Covid-19.
Women should discuss the benefits and risks of having the vaccine with their healthcare professional and reach a joint decision based on individual circumstances.
Women who are breastfeeding can be given the vaccine.
There are no specific safety concerns with the vaccines - but they were not tested on pregnant women during the trials.
Pregnant women are likely to be low down the list of priority groups because of their age, and may only be offered a vaccine in the second phase in 2021.
Can I pay to be vaccinated sooner?
No - this vaccine is being rolled out free to people via the NHS.
You can't jump the queue by paying for it but there should be plenty of vaccine to go round.
Should I leave a gap between getting the flu and Covid vaccines?
If you're eligible for a flu vaccine, you should get it as soon as possible, particularly if you will also be in a high-risk priority group for a Covid jab.
Having both illnesses at once this winter could be dangerous.
At its last meeting, the Joint Committee on Vaccination and Immunisation (JCVI) recommended leaving at least seven days between the vaccines.
What are the side effects?
Some people have mild symptoms, such as muscle aches or a bit of a temperature, after being vaccinated.
This is not the disease itself, but the body's response to the vaccine.
Allergic reactions to vaccines are rare. For any approved vaccine, the ingredients will be listed.
What about other Covid vaccines?
Russia has been using another vaccine, called Sputnik, and the Chinese military has approved another one made by CanSino Biologics.
Both work in a similar way to the Oxford vaccine.

Here are some of your questions about the roll-out of the vaccination, new international travel rules and school closures.
