Family challenge formula milk firm after baby's illness
 Family
FamilyThe parents of a baby who had hospital treatment say he became ill after being fed formula milk from batches which have been recalled by its maker.
A doctor told the family the nine-month old's sickness may have been connected to the milk, but could have been a bug.
Lennie, of Solihull, is one of two children the UK Food Standards Agency (FSA) is aware of after a recall of specific Nutramigen batches.
Manufacturer Reckitt said there was no medical evidence of contamination.
The company recalled batches of two of its products last month - Nutramigen stage 1 and stage 2 hypoallergenic formula powders - over the possible presence of a bacteria which can cause fever and diarrhoea.
The milk feed is often given to babies with a cow's milk protein allergy.
Bacteria Cronobacter sakazakii can also lead to sepsis or meningitis in severe cases.
Reckitt said there was no medical evidence to support the presence of Cronobacter and it had taken the precaution after bacteria had been found in an "isolated overseas sample", which the BBC has now established was in Israel.
 Family
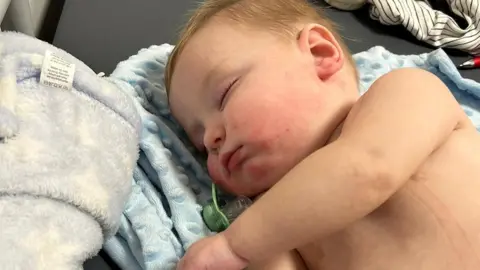
FamilyDaniel said he had called 111 after his son Lennie had been lying grey and still and had been violently sick with extreme diarrhoea on 13 January.
A doctor sent an ambulance to assess the baby who was taken to Birmingham's Heartlands Hospital, where his blood sugar levels were found to be low from fluid loss.
When Lennie improved, the family returned home. They say they were told by a doctor not to use the same batch of Nutramigen formula.
The parent said his son had again been extremely ill on 16 January and needed six outfit and bedding changes during the night.
'My wife was inconsolable'
When they checked, they discovered they had fed their baby a second batch of the formula that had been recalled.
In a letter to Reckitt, the father wrote: "My wife was inconsolable.... having checked our supplies we now calculated that Lennie had consumed in total 24 tubs of the two batches, God only knows how many more in the past that we had disposed of."
The UK recall followed one in the US on 30 December by the US Food and Drug Administration of several different batches from the same manufacturing period.
 Reckitt
ReckittThe family say they have two of the three batches that form part of the FSA recall.
A spokesperson for Heartlands Hospital said: "As the parents had discovered the recall and... changed the milk, the symptoms did settle.
"So if the symptoms return on the milk at any time, the GP has been requested to get a stool sample."
Daniel said Lennie had been poorly intermittently since August, including while on holiday in Spain, prompting multiple trips to the GP and three hospital admissions.
'Sleeping at night'
Since 16 January and after getting rid of the batches, he said Lennie had been a different child.
"He is happy, he has been sleeping at night. He seems more alert, more agile," the father said.
"I can't argue with that, I am seeing it with my own eyes."
When the FSA was approached concerning Lennie's case, it told the BBC it was aware of another baby, a girl, whose illness had been reported to it.
Rajwinder Ubhi, head of incidents, said: "We extend our best wishes to the family.... and hope that she has now fully recovered from her illness."
The recall by Reckitt was voluntary and precautionary, the FSA said.
"Our advice remains that batches ZL3FAA and ZL3F7D and ZL3FDM of this product should not be fed to babies and the product should be returned to the place of purchase."
Reckitt said it was aware of the situations surrounding the two children in the UK who had experienced gastrointestinal symptoms that were "understandably concerning for their parents".
'Safety our absolute priority'
"We wish for their continued recovery. The health and safety of infants is our absolute top priority," it said.
"Reckitt is committed to doing a complete and thorough investigation to understand whether there is any link between their symptoms and their feeding regimen.
"But despite several requests [to families], we have not yet received details to enable a medical investigation.
"As such, we are unable to establish causality or a root cause."
The company said all its products passed extensive testing prior to release.
This included the three batches of Nutramigen formula that were voluntarily recalled from the UK market on 3 January, it added, which all tested negative for Cronobacter.
"Our quality tests and checks are industry-leading and ensure that all products meet or exceed standards set by regulatory bodies."

Follow BBC West Midlands on Facebook, X and Instagram. Send your story ideas to: [email protected]
