Covid: Germany approves AstraZeneca vaccine for over-65s
 Reuters
ReutersGermany's vaccine commission has approved the use of the Oxford-AstraZeneca jab in people aged over 65.
The country previously approved it for under-65s only, citing insufficient data on its effects on older people.
That led to public scepticism about its effectiveness, with some Germans spurning it and leaving many doses unused.
But German Chancellor Angela Merkel said recent studies had now provided enough data to approve it for all ages.
Announcing the commission's decision on Thursday, Health Minister Jens Spahn said the move was "good news for older people who are waiting for an injection".
"The new data also shows that the vaccine is even more effective when the first and second jabs are administered 12 weeks apart," the minister said, adding that the law would be updated to incorporate the new recommendations.
Mrs Merkel said on Wednesday that Germany would follow the UK example of stretching the interval between vaccine doses in order to offer as many people as possible an initial shot.
Various studies have shown the AstraZeneca vaccine is highly effective among the elderly.
Belgium says it will also approve the vaccine for older people, following France earlier this week.
About 5% of Germans have so far received a first vaccine shot.
What led to scepticism about the AstraZeneca jab?
The EU's medical regulator approved the use of the Oxford-AstraZeneca vaccine for all age groups in January.
But the rollout was met by some public scepticism after regulators in countries including France, Belgium and Italy recommended that it should not be used for people over 65. Like Germany, they citied insufficient data on its efficacy for older people.
In January French President Emmanuel Macron said the jab was "quasi-ineffective" for older age groups - a suggestion strongly refuted by the UK government and British medical regulators.
The debate came amid an acrimonious dispute between AstraZeneca and the EU over vaccine supplies to member states.
Since then, stocks of the Oxford-AstraZeneca jab have gone unused in several European countries, slowing vaccination efforts.
Meanwhile the UK - which approved the Oxford-AstraZeneca jab for all age groups - has offered a first dose of a vaccine to 20.7 million people, almost a third of its population.
Last week Carsten Watzl, head of the German Society for Immunology, urged his country to change its mind on the Oxford-AstraZeneca vaccine.
In an interview with the BBC, he predicted regulators would have to reverse their decision to not recommend the jab for older people and suggested Mrs Merkel should take the vaccine live on TV to prove it is safe.
How is Germany planning to ease restrictions?
Separately, Mrs Merkel said she had agreed to a phased easing of restrictions with leaders of Germany's federal states but added that there was the option of an "emergency brake" if case numbers got out of control.
"We are at the threshold of a new phase of the pandemic that we can go into not carelessly but still with justified hope," Mrs Merkel said.
From Monday up to five people from two households will be allowed to meet, with children under 14 exempt.
Some shops will reopen provided that regional case numbers are below 50 per 100,00 people. If cases rise over 50 per 100,000 then customers will have to book slots to visit shops. If cases rise to over 100 per 100,000 over three days in a row then restrictions will be reimposed.
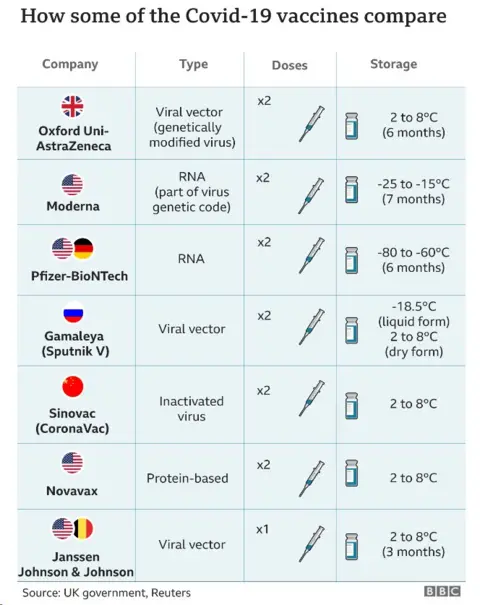
What do we know about the AstraZeneca vaccine?
The UK has been using the vaccine made by AstraZeneca, a UK-Swedish pharmaceutical firm, in its mass immunisation programme since December.
UK health officials say it provides "high levels of protection" for all ages.
No one who received the vaccine in trials was admitted to hospital or became seriously ill with Covid-19.
The vaccine is given via two injections to the arm, the second between four and 12 weeks after the first.