Why India's doctors differ on Covid-19 plasma therapy
 Getty Images
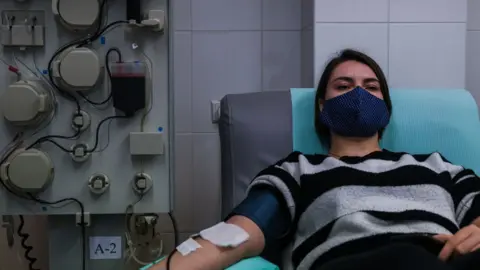
Getty ImagesDesperate calls for blood plasma to treat Covid-19 patients continue to appear on India's social media platforms even as doctors have mixed views about the experimental therapy.
When people have Covid-19 or other viral diseases, their immune system responds by creating antibodies, which attack the virus. Over time the antibodies build up and can be found in plasma - the liquid portion of the blood.
India's health authorities, like many around the world, have allowed the use of plasma to treat severely ill patients as Covid-19 continues to claim lives. The therapy also requires the consent of patients and their families.
But doctors and researchers remain divided over its efficacy. The Indian Council of Medical Research (ICMR) recently warned against its indiscriminate use.
Its own study found that the therapy was not associated with stopping patients from becoming severely ill or reducing mortality.
Several studies around the world have reported similar findings. But the ICMR stopped short of banning the therapy.
 Getty Images
Getty Images"That shows that we can't discard plasma therapy yet," Dr Naresh Trehan, founder of Medanta Hospital, told the BBC. His hospital was one of the early adopters of the therapy in India and "has reported satisfying results".
Dr Trehan says that observational data from Medanta suggests that timing is a crucial factor in the therapy's success.
"Given at a time when a patient is between stage two and three of the cytokine storm, the therapy can be useful. It doesn't work when given in the late stage of the disease," he says.
A cytokine storm happens when Covid-19 sends the body's immune system into overdrive, triggering a self-destructive overreaction. "I would say the jury on plasma therapy is not home yet," he adds.
Dr Om Shrivastava, an infectious diseases specialist at Mumbai's Jaslok Hospital, agrees. His hospital was part of the ICMR's study.
But he also believes that it's too early to discard plasma therapy as major studies around the world have had differing results.
 Reuters
Reuters"My own observational data suggests that the therapy stopped many patients from worsening," he says. He also points to the importance of timing.
The ICMR trial looked at giving plasma to moderate to severe patients, which included patients in ICUs and on ventilators. But Dr Shrivastava says he follows different indicators such as identifying patients who are likely to worsen.
"We don't have to wait for complications to happen before giving plasma therapy. I am pre-empting them," he says.
Some researchers have also pointed out that most studies conducted in the early part of the pandemic, including the ICMR's, did not take into account neutralising antibodies.
Neutralising antibodies are those that stick to the coronavirus and prevent it from infecting other cells. But studies have shown that all recovered patients do not have the same levels of neutralising antibodies.
Scientists believe that the studies that tested the plasma of recovered patients for neutralising antibodies can be considered more reliable.
 Getty Images
Getty Images"The levels and potency of these antibodies differ among individuals and the same must be accounted for in the plasma-therapy trials," says Dr Archita Mishra, a researcher at the Singapore Immunology Network.
This test is expensive and time-consuming, but Dr Mishra adds that it's crucial to explain the discrepancies observed in plasma therapy trials worldwide.
Other factors like age are also important.
A recent study at Johns Hopkins Bloomberg School of Public Health concluded that sex, age and severity of disease may be useful in identifying Covid-19 survivors who are likely to have high levels of antibodies that can protect against the disease.
A recent trial in India also concluded that plasma therapy was effective in mitigating hypoxia (low blood oxygen) and reducing hospital stay.
But a significant section of scientists and doctors worldwide have also reported that the therapy was ineffective.
A study published in the New England School of Journal of Medicine said that no significant differences were observed in clinical status or overall mortality between patients treated with convalescent plasma and those who received a placebo.
The same conclusion has been echoed by several other studies.
Critical care expert Dr A Fathahudeen, who has treated hundreds of Covid-19 patients, believes that the therapy hasn't shown any significant positive impact.
 Getty Images
Getty Images"The therapy initially received a lot of attention because so little was known about the virus and treatment protocols. So, plasma therapy came as a sign of hope and regulators were fine with allowing low-risk experimental treatment protocols," he said.
But a lot has changed since then, he says, adding that plasma therapy is no longer considered to be an effective treatment for Covid-19.
Dr DJ Christopher, head of pulmonary medicine at Christian Medical College in Tamil Nadu state, also does not advocate using plasma therapy on Covid patients.
"My assessment of the available published data is that there is no proof of the efficacy of plasma therapy," he added.
Such starkly differing results and views probably explain why regulators around the world have not dropped plasma therapy from Covid-19 treatment protocols.
Officials in the US have allowed physicians to continue using plasma as an investigational therapy, and more research is being conducted in other countries.
Epidemiologist Dr Chandrakant Lahariya says many reputed studies have conclusively proven that plasma therapy is not effective and it's not a magic bullet.
But, he adds, there are still some answered questions and grey areas and that is why the therapy hasn't been discarded. There is no international protocol on how many neutralising antibodies are necessary to qualify as a donor or if a test to determine the presence of such antibodies should be mandatory.
"Also, there is no effective or proven treatment yet and that's probably the reason why plasma therapy is continuing," Dr Lahariya added.
Dr Mishra agrees.
"The studies around the world suggest no significant side-effects of plasma therapy so far, so the potential benefits clearly outweigh the risks at the moment," she added.

Read more stories by Vikas Pandey

