Cystic fibrosis: Drug plea for woman 'struggling to breathe'
 Ciaran McVarnock
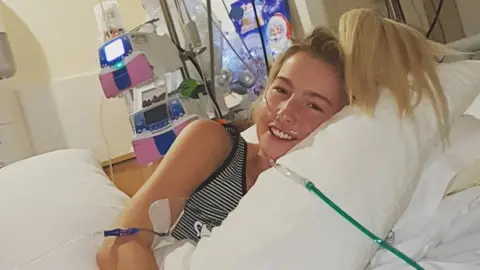
Ciaran McVarnockThe partner of a 28-year-old woman who has been "struggling to breathe" due to cystic fibrosis has pleaded for her to be given new NHS-approved medication.
Nicole Adams is in Belfast's Royal Victoria Hospital where her lung capacity recently fell to below 20%.
Her partner Ciaran McVarnock told BBC News she had to fight for her life but is now showing signs of improvement.
He is calling for her to be given access to Symkevi, a drug recently passed for NHS use in Northern Ireland.
"We've been told it is available but we're not being told why Nicole's not getting it," he said.
Cystic fibrosis is a genetic condition that can cause fatal lung damage.
About half of patients with the condition die before the age of 32.
'Life support'
Ms Adams, from Newtownabbey, County Antrim, recently had to give up her hairdressing business after a deterioration in her health.
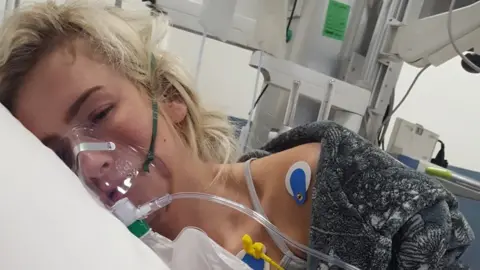
Mr McVarnock said his partner was admitted to hospital just over two weeks ago and at one point was told she may need life support in the intensive care unit.
 Ciaran McVarnock
Ciaran McVarnock"It was probably the saddest moment of my life," he said.
He suspects Ms Adams may still be too weak to begin new treatment at the moment, but wants reassurance that Symkevi supplies are ready and available when she is strong enough.
"She's hanging on, she's fighting, but the thing is Nicole hasn't got time. Anyone with cystic fibrosis - they haven't got time," he said.
Medical treatments are available which can improve lung function but they are expensive and do not work for every patient.
In recent weeks, several life-extending medications became available on the NHS after health authorities in different parts of the UK struck separate deals with drug manufacturer Vertex Pharmaceuticals.
The best known of these medications is Orkambi, but Vertex also produces Symkevi, which is restricted to patients over 12 years of age and Kalydeco, which can be used from 12 months.
In September, the Scottish government reached a five-year agreement with Vertex Pharmaceuticals to make Orkambi and Symkevi available to eligible patients in Scotland.
In October, NHS England announced it would make Orkambi; Symkevi and Kalydeco available to its population within 30 days.
'Clinically appropriate'
In recent weeks, Northern Ireland's Department of Health has followed suit and concluded a deal with Vertex Pharmaceuticals.
The department confirmed to BBC News NI on Friday it has an agreement currently in place to supply Orkambi, Symkevi and Kalydeco to cystic fibrosis patients in Northern Ireland.
"Doctors can now assess patients and provide the drugs to patients when deemed clinically appropriate," a statement from the department said.
It did not say why Ms Adams has not yet been offered Symkevi.
The Belfast Health Trust, which is treating her in the Royal Victoria Hospital, said: "We cannot comment on the treatment of individual patients."
The trust added a number of its patients were "being clinically assessed for commencement of CFTR modulators".
CFTR is short for "cystic fibrosis transmembrane conductance regulator" and is the term used to refer to drugs like Orkambi which aim to improve lung function by targeting genetic mutations.
Mr McVarnock said his partner had a very rare form of cystic fibrosis and about 18 months ago was given access to Orkambi on "compassionate grounds" due to poor health.
 Ciaran McVarnock
Ciaran McVarnockHe explained she suffered a bad reaction to the drug and had to stop the treatment.
"What they should have done is try the next option," he said, arguing that Ms Adams should have been offered a Symkevi trial at that time.
He is also campaigning for her to be allowed to try a new triple combination therapy drug, Trikafta, on compassionate grounds.
Trikafta was recently approved for patient use in the United States but it is not yet licensed in the UK.
The drug is also produced by Vertex Pharmaceuticals and the couple have contacted the company for help accessing supply but have not received a reply.
'Specific eligibility'
BBC News NI asked Northern Ireland's health authorities about patients' access to Trikafta on compassionate grounds.
In a statement, the Health and Social Care Board said: "Use of medicines on a compassionate basis may be undertaken following agreement between the patient's consultant and a pharmaceutical company.
"The board understands that for a patient to receive a medicine on a compassionate basis they may first need to meet specific eligibility criteria as set out by the manufacturer.
"The board does not have a role in the decision-making process for a patient to access a medicine on a compassionate basis."
Mr McVarnock has contacted several news outlets in a bid to help his partner access treatment and continues to support her while she is in hospital.
Allow X content?
"It's really hard. There are times that you have to go out to the toilet just to cry," he said.
"Nicole is a really lively person, she's just a real lovely, fun character and to see her like this, it brings everybody else down.
"It's so sad, so hard to see."
