Charlie Gard: Mother says terminally-ill son 'not in pain and suffering'
 PA
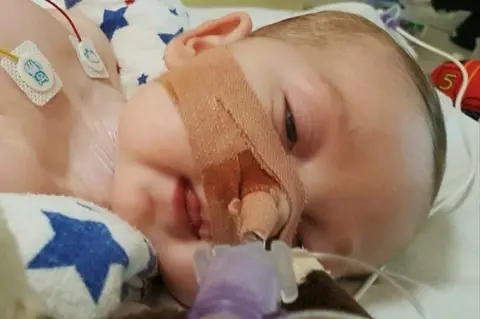
PAThe mother of terminally-ill Charlie Gard has said he is not in "pain and suffering".
It comes after a US hospital offered to ship an experimental drug to the UK to help treat him.
It also offered to admit the 11-month-old if "legal hurdles" can be cleared. Great Ormond Street hospital has said further treatment will not help.
Foreign Secretary Boris Johnson has said it would be impossible for Charlie to be transferred to another hospital.
Charlie's mother Connie Yates told Good Morning Britain on Friday: "We are not bad parents, we are there for him all the time, we are completely devoted to him and he's not in pain and suffering, and I promise everyone I would not sit there and watch my son in pain and suffering, I couldn't do it."
 PA
PAMs Yates said the Pope's intervention earlier this week came after she wrote a letter to him.
She said: "It does give us a hope definitely, because there was no hope left. Charlie was going to die on Friday and, you saw the video we did, we were absolutely devastated.
"We had no control over it, the way it was done.
"And then it was going to be on the Monday instead but I think the White House got involved over the weekend and then that changed things."
Charlie has mitochondrial depletion syndrome, a rare genetic condition which causes progressive muscle weakness.
Doctors have said he cannot see, hear, move, cry or swallow and that his life support should be switched off because there is no chance of his condition improving.
Charlie's parents, Ms Yates and Chris Gard, raised £1.3m on a crowdfunding site to pay for experimental nucleoside therapy in the US.
But they lost a legal battle with the hospital last month after judges at the European Court of Human Rights ruled further treatment would "continue to cause Charlie significant harm".
Drug shipment
The US hospital, which cannot be named for legal reasons, said that it would treat the boy with an experimental drug pending approval from government regulators, the Food and Drug Administration (FDA).
It said it had "agreed to admit and evaluate Charlie, provided that arrangements are made to safely transfer him to our facility, legal hurdles are cleared, and we receive emergency approval from the FDA for an experimental treatment as appropriate".
It added: "Alternatively, if approved by the FDA, we will arrange shipment of the experimental drug to Great Ormond Street Hospital and advise their medical staff on administering it if they are willing to do so."
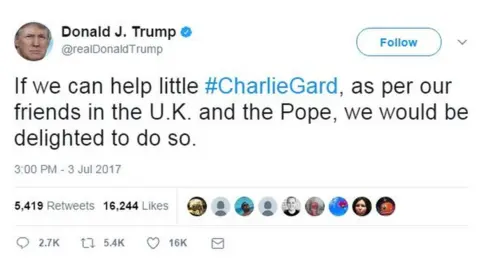 Twitter/ @realDonaldTrump
Twitter/ @realDonaldTrumpA US specialist told judges that a "small chance" of a meaningful improvement in Charlie's brain function would be provided by therapy.
Charlie's parents, from Bedfont, west London, have spent the last days of their son's life with him, after being given more time before his life-support is turned off.
Last week they said the hospital had denied them their final wish to take their son home to die.
