Antibody jab approved for common winter virus RSV
 Christine Burlison
Christine BurlisonA new treatment to protect babies against a common and potentially dangerous winter virus has been approved by the UK regulator.
The single antibody shot helps stop infants getting chest infections, such as pneumonia, for about six months.
A large study has now begun to find out whether nirsevimab should be offered to all babies in the UK.
Respiratory syncytial virus (RSV) is one of the main reasons children under five end up in hospital.
Other treatments for RSV already exist and trials of a vaccine have also shown promise.
In a normal winter, RSV mostly causes coughs and colds which clear up in a couple of weeks - but it can be particularly serious in infants under the age of two, causing severe lung problems such as bronchiolitis and pneumonia.
Every year, about 29,000 babies need hospital care for RSV and most have no other health issues beforehand.
Many infants now have little natural protection from the usual winter viruses. They were born during the pandemic when lockdowns and restrictions helped suppress their spread - and that is concerning health officials.
Although RSV rates are currently higher in the UK than during the pandemic, they are still in line with a normal winter. Levels of RSV are also surging in the US.
 Christine Burlison
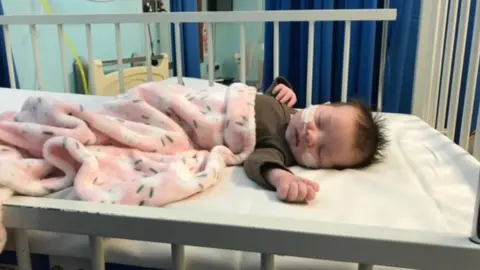
Christine BurlisonChristine Burlison thought her newborn baby Aria had nothing more than a cold when she started sneezing and snuffling not long after coming home.
But then Aria started grunting when breathing, and the next day she suddenly went floppy.
"It was really distressing," Christine says.
"I'd never heard of RSV - it's not part of antenatal training, but I knew something wasn't right."
An ambulance took Aria to hospital where she was given oxygen to help her recovery, and she was there for a week.
Christine says the experience of seeing her tiny daughter struggling to breathe was "heart-breaking" and "so terrifying".
When her second child, Jude, became unwell with breathing problems when he was eight months old, she knew the signs to spot.
"I was really aware of RSV and knew when his breathing had crossed a line," she says.
 Christine Burlison
Christine BurlisonThe new antibody treatment, called nirsevimab, from Sanofi and AstraZeneca, has already been shown to reduce lower respiratory tract infections caused by RSV by 74.5% in trials involving 4,000 babies.
It works by preventing RSV from fusing to cells in the respiratory tract and causing infections.
But it still needs more research in larger numbers of babies before it can be used on the NHS.
Researchers now plan to investigate whether it can cut the number of babies needing hospital care for RSV, and are urging parents to sign up to their study.
The study is open to newborn babies and those up to 12 months old. Only one visit for the antibody injection is needed, and follow-up sessions happen via an app.
Co-study leader Dr Simon Drysdale, consultant paediatrician in infectious diseases at London's St George's Hospital, said the treatment could eventually be given at birth to offer protection for the first months of life, or during routine immunisations at two months old.
That is a decision the NHS and the UK's vaccine committee will have to make, based on the study's results.
"We do need the hospitalisation data to make complicated decisions between different technologies available," said Prof Saul Faust, co-study leader from the University of Southampton.
Another form of preventative treatment, a vaccine against RSV made by Pfizer, has produced promising results in trials in the US.
Given to pregnant women to protect their newborns, trials show the jab was 81% effective against severe respiratory illness because of RSV in the first 90 days of a baby's life.
It also showed 69% protection for the first six months of a baby's life.
Pfizer plans to start the process of getting approval from medicines regulators by the end of this year.
Vaccines prompt the body to make antibodies to protect it against RSV, and this can take a month or two to happen.
An injected antibody treatment works straight away to protect against RSV.
An antibody treatment called palivizumab already exists for infants in the UK, but it has to be injected once a month for five months.