Spinal muscular atrophy: Gene therapy approved by NHS
 Science Photo Library
Science Photo LibraryA life-changing drug has been approved by the NHS to treat babies with a rare fatal genetic disorder.
The gene therapy Zolgensma offers hope to infants with a type of severe spinal muscular atrophy (SMA).
With a list price of £1.79m it could become the most expensive drug ever approved by the National Institute for Health and Care Excellence (Nice).
NHS England says it has negotiated an undisclosed discount that is 'fair to taxpayers'.
The Scottish Medicines Consortium also announced later on Monday that it has approved the drug.
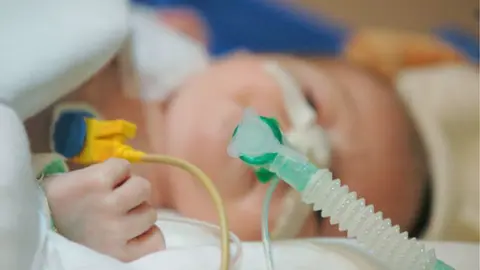
Around 80 babies and young children with type 1 SMA could benefit from the treatment each year in England, say experts.
The condition causes muscle weakness and affects movement and breathing, meaning most babies who have it do not live past two years without intervention.
Children with the condition often need breathing assistance or a feeding tube.
The one-off treatment has been described in the British Medical Journal as "the most expensive drug course of treatment ever" .
In draft guidance, Nice ruled that although the drug was hugely expensive, its use is justified by the exceptional impact it can have on young children.
It is hoped that if the drug is given to children in their first six months of life before they develop symptoms, it might come close to being a cure by halting disease progression.
 Rajdeep Patgiri
Rajdeep PatgiriRajdeep Patgiri's daughter Tora had the treatment in the US when she was nearly 11 months old.
Rajdeep says it has made a big difference to her life and what she can now do: "She has been able to sit independently...she can stand independently and walk with assistance. So a lot of progress.
"Before, at the time of diagnosis and before treatment, time felt like an enemy because every day you expect something to deteriorate. And now, time is a friend because every day she improves even by tiny bits so there is always something to look forward to."
The drug contains a replica of the missing gene SMN1.
In studies, it has helped babies to reach milestones such as breathe without a ventilator, sit up on their own and crawl and walk after a single infusion treatment.
At present only limited data about the impact of Zolgensma is available from clinical trials, so any decision to give it to older children aged between six and 12 months will have to be taken by a national team of clinicians.
Another drug called Spinraza, which is already available, can help slow the degeneration in some SMA patients, but it must be taken for life.
Meindert Boysen, deputy chief executive and director of the Centre for Health Technology Evaluation at Nice, said: "Spinal Muscular Atrophy is a very serious, debilitating and distressing condition that has very significant effects on every aspect of life of those with SMA, and their families and carers.
"As is the case with many new treatments for very rare diseases, limited evidence means there are uncertainties about the long-term benefits.
"But for some babies who are diagnosed before they have symptoms, it might come close to being a cure."
NHS England Chief Executive Sir Simon Stevens said they had "moved mountains" to make the treatment available.
