Cannabis meds: 'I risk criminal record to help my child'

Anthony Clarry has had to get used to breaking the law. Once a month he smuggles two cannabis-based medicines into the UK for his five-year-old daughter Indie-Rose, who has a rare form of severe epilepsy.
Speaking minutes after clearing customs at Stansted Airport, he told the BBC: "Every time I come back from the Netherlands I am really anxious that they might stop me and then I risk a criminal record, and also having Indie's medicine taken away which would potentially put her life at risk."
Indie-Rose's mother, Tannine Montgomery, has also made the trip. She said: "We should not have to spend £1,500 a month on these medicines, but be able to pick it up from a local pharmacy."
At home in Clare, Suffolk, they are reunited with Indie-Rose, who has Dravet syndrome, a rare and hard to treat form of epilepsy.
They say the cannabis oils have dramatically reduced the frequency, duration and severity of their daughter's seizures.
Tannine said: "Since she has been on the cannabis oils, she has not been hospitalised with a seizure. Not only that, but she is more alert, happier, a different child, and it's made her life worth living."
The couple have spent about £25,000 on cannabis medicines over the past year.
Much of that has been raised through crowdfunding online, and with the support of their local community.
The whole family had to spend several weeks in the Netherlands while Indie-Rose was assessed by a Dutch doctor, who has prescribed two cannabis-based oils, Bedrolite and Bedica.
But wasn't the cannabis meds law changed in 2018?
It was. On 1 November 2018, cannabis medicines were moved from Schedule 1 of the Misuse of Drugs Regulations, meaning they have no therapeutic value, to Schedule 2, to recognise there is conclusive evidence of benefit for some patients.
From that date, specialist doctors were allowed to prescribe cannabis medicines "where there is an unmet clinical need". following a recommendation from the Chief Medical Officer Dame Sally Davies.
But Tannine and Anthony have not been able to persuade their daughter's neurologist to prescribe the Dutch products, so they have to pay for it.
The campaign group End Our Pain says there are several other families who are bringing cannabis medicines into the UK illegally.
There are no official figures, but it appears that only two children with severe epilepsy now have NHS prescriptions for unlicensed cannabis medicines.
 Fergus Walsh/BBC
Fergus Walsh/BBCOne of them is Sophia Gibson, aged seven, from Newtownards near Belfast.
She also has Dravet syndrome and used to suffer life-threatening seizures.
Her mum, Danielle Davis, told the BBC: "Barely a week went by without us having to call an ambulance for Sophia, because her seizures were impossible to control.
"Sometimes the doctors had to put her into an induced coma and take over her breathing, it was terrifying."
Danielle says the cannabis medicines have had a dramatic impact.
"Sophia has not been hospitalised as a result of a seizure since last July. We know it is not a cure because she still has seizures, but they are infrequent, last less than a minute and are much milder.
"She is also happier, more alert and her cognitive ability has improved," her mum says.
What is in the cannabis-based medicines?
There are hundreds of chemicals in cannabis. The two key active compounds, called cannabinoids, in medical use are:
- tetrahydrocannabinol (THC) - which is the main psychoactive, mood-altering component in cannabis
- cannabidiol (CBD) - which is not a controlled substance
A variety of CBD oils can be bought in the UK, but only if they contain virtually no THC.
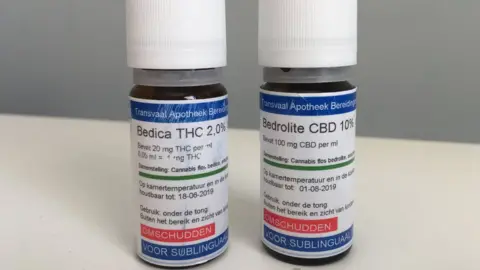
Indie-Rose and Sophia are being treated with Bedrolite, and Bedica, which are administered under the tongue.
Bedrolite is 9% CBD and less than 1% THC, but still above the 0.2% legal limit in the UK.
Bedica is 14% THC.
 Fergus Walsh
Fergus WalshThey are manufactured in the Netherlands by Bedrocan, whose sole customer is the Dutch government.
The dried cannabis flowers are turned into oil-based medicines by a pharmacy in The Hague.
The British Paediatric Neurology Association (BPNA) guidelines say there is "good quality clinical evidence" that CBD reduces seizures in Dravet syndrome, but "no high quality evidence" to support the use of THC.
So why can't more children with severe epilepsy access cannabis medicines under the new law?
This is a key question.
Last year, Home Secretary Sajid Javid said: "Having been moved by heartbreaking cases involving sick children, it was important to me that we took swift action to help those who can benefit from medicinal cannabis.
"We have now delivered on our promise and specialist doctors will have the option to prescribe these products where there is a real need."
Understandably, politicians recognised that clinical decisions had to be left to doctors, but paediatric neurologists have largely refused to prescribe cannabis-based medicines from companies such as Bedrocan in the Netherlands or Tilray in Canada.
Why?
Prof Helen Cross, a consultant in paediatric neurology at Great Ormond Street Hospital, and a leading epilepsy researcher, said: "There was a perception from families, after 1 November, that they could walk in and get a prescription for cannabis-based medicines, which they perceive to be a natural product, so must be better than other drugs.
"But we need to look at the evidence base and ensure we are not going to make the children any worse."
At present there is an impasse because the BPNA says there is not enough evidence that THC is safe or effective, and it has concerns about its effects on the developing brain.
Later this year, the health watchdog the National Institute for Health and Care Excellence (NICE) will issue guidelines to specialist doctors which may clarify the circumstances in which products containing THC can be prescribed.
Prof Cross accepts some children on these medications may be doing well, but not everyone.
"I've seen an equal number of very disappointed families, because they haven't seen the miracle that they're expecting, but these cases don't get reported, as it's not what people want to hear."
Sophia and Indie-Rose's parents say some of the standard epilepsy medicines left the girls heavily sedated and lethargic, and did not control their seizures.
Prof Cross is hopeful that a new cannabidiol medicine, Epidiolex, manufactured in the UK by GW Pharma, will get a European licence within a few months. It contains no THC.
Epidiolex has undergone randomised controlled trials, and was found to reduce seizures by nearly 40% in children with Dravet or Lennox Gastaux syndromes.
About 80 children in the UK are already being prescribed Epidiolex on a compassionate basis, where their seizures have proved resistant to other medications.
Prof Cross, who led the trials of Epidiolex in the UK, said it was sensible to start with cannabidiol and then see "do we need to add THC in some circumstances?".
But it won't come cheaply. The list price of the drug in the US is $32,500 (£25,000) a year.
There has been a broad welcome from clinicians and parents for the decision to move cannabis medicines from Schedule 1 to Schedule 2, as this will make research much easier.
Prof Cross says she hopes to set up clinical trials involving some of the unlicensed cannabis medicines which will seek to establish whether THC has a beneficial role in controlling seizures.
Follow Fergus on Twitter.
