Covid: What is happening with the EU vaccine rollout?
 Reuters
ReutersThe rollout of Covid vaccines in European Union (EU) countries is picking up speed, with more than 300 million jabs administered.
As of 20 June, nearly half of the EU's adult population have had at least one one dose, while 28% have been fully vaccinated.
Earlier this year, the rollout was hit by delays in production and distribution and vaccine hesitancy in some countries.
How well are EU countries doing?
In the week to 20 June 2021, Germany administered an average of 1 dose per 100 people a day, with Italy and France close behind on 0.9 dose per 100 people - all higher than the UK's 0.6 per 100.
Hungary - which is using Russian and Chinese vaccines as well as the EU-approved ones - has fully vaccinated 46% of its population, the same proportion as the UK.
In France, children who are 12 and over can be vaccinated, with parental consent. The government is hoping to avoid class closures when schools reopen after the summer holidays.
The EU authorised the use of the Pfizer vaccine in children aged 12-15 at the end of May.
Germany said it would give it to those aged 12-17 with pre-existing conditions while a number of other EU countries said they would vaccinate children before the start of the next academic year.

Initial problems with the rollout
In June 2020, all 27 member states joined a scheme giving the EU central responsibility for buying vaccines.
However, the EU was slower than the UK to negotiate a contract with AstraZeneca and there were supply problems.
Its deals with Pfizer-BioNTech and Moderna also saw early problems with production and distribution.
In February, European Commission President Ursula von der Leyen acknowledged the EU's vaccine difficulties, saying: "We were late to authorise. We were too optimistic when it came to massive production and perhaps too confident that what we ordered would actually be delivered on time."
Issues with AstraZeneca
In January 2021, the European Medicines Agency (EMA) approved the use of the Oxford-AstraZeneca vaccine for all age groups, but a number of EU countries initially refused to recommend its use for people over 65.
France and Germany eventually revised their stance and approved the vaccine for 65-74 year olds at the beginning of March.
They were again among 13 European countries who paused the AstraZeneca rollout again later in March, following reports that a small number of people developed blood clots after receiving the jab.
On 7 April, the EMA said there was a "possible link" between the vaccine and the clots, although it is still recommended the jab for all age groups.
Several EU countries, including France, Germany and the Netherlands now say only people over 55 or 60 should get the jab, while others like Denmark have suspended its use altogether.
The headlines surrounding AstraZeneca led to a drop in confidence in the jab. In March, the polling company YouGov suggested that only a third of Germans and 23% of French respondents considered it safe.
 Reuters
ReutersThe EU took AstraZeneca to court to force it to deliver more doses of vaccine. The company blamed production problems but said it had "fully complied" with its contract.
The EU lost its legal battle. It had wanted AstraZeneca to deliver 120 million doses by the end of June but a court ruled that the company must instead provide 80 million doses by the end of September.
The vaccine exports row
The EU said the UK has had an unfair advantage in contracts it signed with vaccine manufacturers, some of whom have plants in the EU.
EU leaders considered a ban on exports of vaccines to the UK but decided not to introduce one.
Instead, they called for more transparency from the UK and other countries on the number of doses they export.
The EU says that by 1 June 2021, it had exported 245 million doses to 46 different countries.
Can EU states make their own deals?
Under the terms of the EU scheme, member states are not supposed to strike deals with any vaccine manufacturer with whom the EU already has an agreement, but they can make separate deals with vaccine makers which have not signed agreements with the EU.
Hungary has been using Russia's Sputnik vaccine for months and and Slovakia has also bought doses of it.
Germany said in April it would begin talks to secure supplies of the Russian vaccine and Austria said it would buy one million doses, but only once the EU's medicines agency approves the jab for use in the EU.
The Czech Republic and Poland have entered talks with both Russia and China.
Did the UK roll out vaccines quicker because of Brexit?
The UK approved the Pfizer vaccine in November 2020, nearly three weeks before EU regulators.
The government claimed that being outside the EU allowed it to be more nimble in this area.
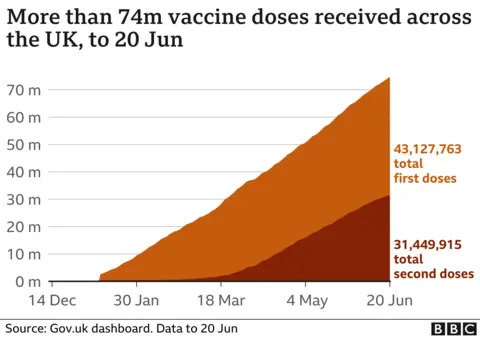
However, the UK's approval of the vaccine would have been permitted anyway under EU law - a point made by the head of the UK medicines regulator.
The UK could have joined the EU vaccine scheme last year while it was still in a transition phase with the EU, but it chose not to.
If it had, the UK might not have been able to do as many deals with vaccine companies.

- EASY STEPS: How to keep safe
- A SIMPLE GUIDE: What are the symptoms?
- CONTAINMENT: What it means to self-isolate
- HEALTH MYTHS: The fake advice you should ignore
- MAPS AND CHARTS: Visual guide to the outbreak

