New drug could help women avoid ectopic pregnancy surgery
 University of Aberdeen
University of AberdeenResearchers in Scotland are trialling a new drug that could help women with ectopic pregnancies avoid emergency surgery.
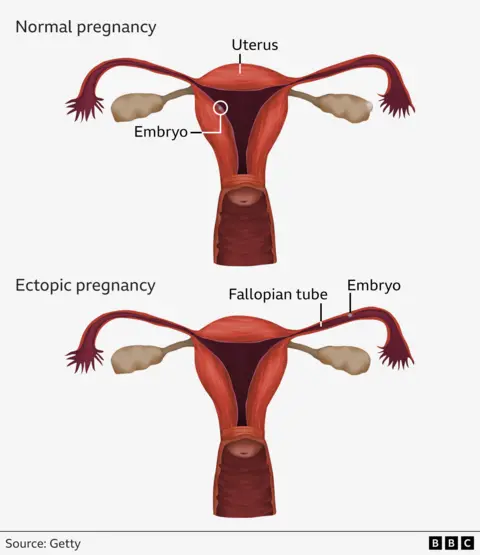
About one in 90 pregnancies are ectopic - which means the embryo starts growing outside the womb, usually in the fallopian tube, and can be life-threatening.
About 40% of women can be treated with medication, but the current drug does not work in about 30% of cases - meaning they have to receive surgery to remove their fallopian tube.
The hope is that the latest drug, trialled by the University of Aberdeen, will work for more women and reduce the number of operations needed.
If so it could be the first medical advancement in ectopic pregnancies in about 20 years.
How are ectopic pregnancies treated?
In the UK there are about 11,000 ectopic pregnancies per year. These experiences can have a significant physical and mental impacts on women.
Because embryos in these cases implant outside of the womb, they cannot develop into babies.
Symptoms for women usually start between four and 12 weeks according to the NHS, and can include pain low down on one side of the abdomen and pain in the tip of the shoulder.
As the embryo grows, there is a risk that the fallopian tube can burst which can lead to life-threatening internal bleeding - this requires emergency surgery to remove the tube partially or fully.
There have been advances in diagnosing ectopic pregnancies which have enabled women to be treated with medication.
Currently patients receive an immune-system suppressant called methotrexate which stops the pregnancy growing in about 70% of cases.
But researchers hope to demonstrate this figure could be improved by also giving women the hormone mifepristone - which blocks the key pregnancy hormone progesterone.
The trial plans to recruit hundreds of women who are eligible for medical treatment across 40 hospitals in the UK.
Some will receive mifepristone alongside methotrexate while others will receive a placebo.
The work will be led by Dr Andrea Woolner, senior clinical lecturer at Aberdeen university.
Dr Woolner said: “Ectopic pregnancy is a devastating pregnancy loss which has significant physical and psychological impacts on women and their families, and the medical management of ectopic pregnancy has essentially had no advances in over 20 years.
“This is a really exciting opportunity to trial an additional drug alongside standard treatment for medical management of ectopic pregnancy."
'I had to learn to live again'
Helen Corsicadmore from Cardiff says it took "years of healing" before she felt ok after experiencing two ectopic pregnancies.
On both occasions, the 43-year-old had to have surgery to remove her fallopian tubes.
 University of Aberdeen
University of AberdeenDoctors attempted to manage her first with methotrexate - but she experienced a rupture on the same day she was treated.
Helen, the co-ordinator at The Ectopic Pregnancy Trust, said: "This was extremely hard for me - I felt like I had to learn to walk and live again all in one go, whilst trying to heal both physically and mentally.
“It took a good few years of healing before I felt ok again. I had never had any surgery previously, let alone any major unexpected surgery, so this was difficult to process both physically and mentally for some time."
The trial is due to start later this year and results are expected in 2027.
It will be funded by the Medical Research Council (MRC) and the National Institute for Health and Care Research (NIHR).
A team of experts will be involved from the universities of Edinburgh, Nottingham and Birmingham, University College London, Monash University, Australia, Imperial College London and The Ectopic Pregnancy Trust.
