The cells that protect your brain against infection could also be behind some chronic diseases
 Serenity Strull/BBC/Getty Images
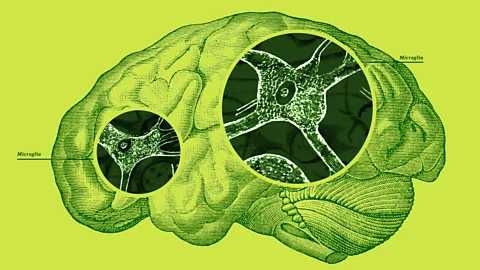
Serenity Strull/BBC/Getty ImagesMicroglia are the brain's resident immune cells. Their job is to patrol the brain's blood vessels looking for invading pathogens to gobble up. But what happens when they go rogue?
Historically they've been overlooked – seen as simple foot soldiers of the immune system. Yet increasingly, scientists believe that microglia may have a more directorial role, controlling phenomenon from addiction to pain. Some believe they may even play a key role in conditions such as Alzheimer's disease, depression, anxiety, long Covid, and myalgic encephalomyelitis (ME), also known as chronic fatigue syndrome.
But what exactly are microglia?
There are two types of cells that make up the brain. Neurons, also known as nerve cells, are the brain's messengers, sending information all over the body via electrical impulses. The other type – glia – make up the rest. Microglia are the smallest member of the glia family and account for about 10% of all brain cells. The small cells have a central oval-shaped "body" from which slender tendril-like arms emerge.
"They have a lot of branches that they are continually moving around to survey their environment," says Paolo d'Errico, a neuroscientist at the University of Freiburg, Germany. "In normal conditions they extend and retract these processes in order to sense what is happening around them."
When performing well, microglia are essential to healthy brain function. During our early years, they control how our brain develops by pruning unnecessary synaptic connections between neurons. They influence which cells turn into neurons, and repair and maintain myelin – a protective layer of insulation encasing neurons, without which transmission of electrical impulses would be impossible.
 Getty Images
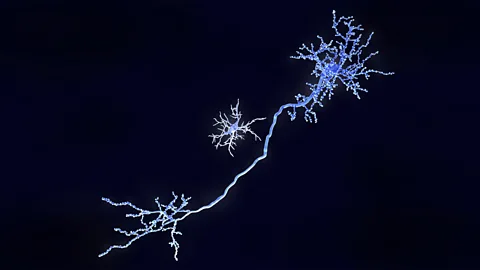
Getty ImagesTheir role doesn't stop there. Throughout our lifetimes, microglia protect our brains from infection by seeking out and destroying bacteria and viruses. They clean up debris that accumulates between nerve cells, and root out and destroy toxic misshapen proteins such as amyloid plaques – the clumps of proteins thought to play a role in the progression of Alzheimer's disease.
Yet in certain circumstances they can go rogue.
"There's two sides to microglia – a good side and a bad side," says Linda Watkins, a neuroscientist at the University of Colorado Boulder.
"They survey for problems, looking for unusual neural activity and damage. They're on the watch for any kind of problems within the brain, but when they get super excited, they turn from being the vigilant good guys to the pathological bad ones."
What makes them go rogue? When microglia sense that there is something wrong in the brain, such as an infection, or a large presence of amyloid plaques, they switch into a super-reactive state.
"They become much larger, almost like big balloons, and they pull in their appendages and start moving around, munching up damage like little Pac-Mans," says Watkins.
Activated microglia also release substances known as inflammatory cytokines, which serve as a beacon, calling other immune cells and microglia to action. Such a response is necessary to help the body fight off invaders and threats. Usually after a certain amount of time, microglia revert back to their "good" status.
However, it appears sometimes microglia can stay in this super-excited state long after the infectious agent has disappeared. These out-of-control microglia are now thought to be behind a variety of modern diseases and conditions.
Take addiction. This condition has historically been viewed as a disorder of the dopamine neurotransmitter system, with imbalances of dopamine being to blame for sufferers' increasingly drug-focused behaviour.
 Getty Images
Getty ImagesBut Watkins has a different theory. In a recent academic article, Watkins and scientists from the Chinese Academy of Sciences argue that when a person takes a drug, their microglia see the substance as a foreign "invader".
"What we found out through our own research, was that a variety of opiates activate microglial cells, and they do so at least in part through what's called the 'toll like receptor' (TLR)," says Watkins.
"Toll like receptors are very ancient receptors designed to recognise foreign objects. They're supposed to be there to detect fungi, bacteria and viruses. They're the 'not me, not right, not okay' receptors."
When microglia detect drugs such as opiates, cocaine or methamphetamine they release cytokines, which causes the neurons that are active at the time of drug-taking to become more excitable. Crucially, this leads to new and stronger connections between the neurons being formed, and more dopamine being released – strengthening the desire and craving for drugs. The microglia change the very architecture of the brain's neurons, leading to drug-taking habits that can last a lifetime.
The evidence to support this theory is compelling. For one, drug abusers have raised inflammation and inflammatory cytokines in the brain. Reducing inflammation in animals also reduces drug-seeking behaviour. Watkin's team has also shown that you can stop mice from continually seeking out drugs like cocaine by blocking the TLR receptor and preventing microglial activation.
Microglia could play an important role in chronic pain too, defined as pain that lasts longer than 12 weeks. Watkins' laboratory has shown that after an injury, microglia in the spinal cord become activated, releasing inflammatory cytokines that sensitise pain neurons.
"If you block the activation of the microglia or their pro-inflammatory products, then you block the pain," says Watkins.
According to Watkins, microglia could even explain another phenomenon; why elderly people experience a sharp decline in their cognitive abilities following a surgery or infection. The surgery or infection serves as a first hit which "primes" microglia, making them more likely to adopt their bad guy status. Following surgery, patients are often given opioids as pain relief, which unfortunately activates microglia again, causing an inflammation storm that eventually causes the destruction of neurons.
The field of research is in its infancy, so these early findings should be treated cautiously, but studies have shown that you can prevent post-surgery memory decline in mice by blocking their microglia.
"If I walk up to you and, without any forewarning whatsoever, slap you across the face, I get away with it the first time. But you don't let me get away with it the second time because you're primed, you're ready, you're on guard," says Watkins.
"Glial cells are the same way. With ageing, glial cells become more and more primed and ready to over respond as the years go by. And so now that they're in this prime state, a second challenge like surgery makes them explode into action so much stronger than before. Then you get opioids, which are a third hit."
 Getty Images
Getty ImagesThis "priming" of microglia could even be behind Alzheimer's disease (AD). The main risk factor for AD is age, and if microglia become more ready to respond as we get older, it could be a factor. At the same time, one of the main hallmarks of AD is the build-up of clumps of amyloid protein in the brain. This process begins decades before symptoms of confusion and memory loss become detectable. One of microglia's jobs is to hunt down and remove theses plaques, so it's possible that over time, repeated activation causes microglia to permanently switch into rogue mode.
"The accumulation in the brain of amyloid induce microglia to became more reactive," says D'Errico.
"They start to release all these inflammatory signals, but the point is that since these amyloid plaques continue to be produced, there is constant chronic inflammation that never stops. This is really toxic for neurons."
Chronically activated microglia can engulf and kill neurons directly, release toxic reactive species that damage them, or start "over-pruning" synapses, destroying the connection between nerve cells. All these processes could ultimately lead to the confusion, loss of memory, and loss of cognitive function that characterises the disease.
In a 2021 study, d'Errico even found that microglia can contribute to the spread of Alzheimer's disease by transporting the toxic amyloid plaques around the brain.
 Getty Images
Getty Images"In the early stages of Alzheimer's there are particular regions in the brain that seem to accumulate plaques, such as the cortex, the hippocampus, and the olfactory bulb," says d'Errico.
"In the later stages of the disease there are many more regions that are affected. We found that microglia are able to internalise the amyloid protein, and then move to another region before releasing it again."
Some of the symptoms of Alzheimer's, such as forgetfulness and loss of cognitive function, are similar to those suffering from long Covid, and it's possible that errant microglia could be behind "brain fog" too. For example, one of the main factors that cause microglia to turn rogue is the presence of a viral infection.
"Abnormally activated microglia may start over pruning of synapses in the brain, and this may lead to cognitive decline, memory loss, and all those symptoms related to the brain fog syndrome," says Claudio Alberto Serfaty, a neurobiologist at the Federal Fluminense University, in Rio de Janeiro, Brazil, who summarised the evidence for this theory in a recent review article.
The hope is that this new way of thinking will eventually lead to new treatments.
For example, clinical trials for new Alzheimer's drugs are currently underway that aim to boost microglia's capacity to destroy amyloid. However, as with all Alzheimer's drugs, such a strategy would work best in the early stages of the disease, before significant neural death has occurred.
For addiction, one idea is to swap the errant microglia that have gone wrong with the "normal" microglia that are present in the brains of non-drug takers. This concept, known as microglia replacement, involves grafting microglia into the specific brain regions by bone marrow transplantation.
However, such an approach would prove difficult. After all, active microglia are necessary to fight off infections; in fact, they're vital for brain function.
"In theory, yes it could work, but keep in mind that you don't want to disrupt microglia all over your brain, it would need to be localised," says Watkins. "Microinjecting microglia into specific brain areas would be very invasive. So, I think we need to look for something that's safe for that kind of a treatment."
--
If you liked this story, sign up for The Essential List newsletter – a handpicked selection of features, videos and can't-miss news, delivered to your inbox twice a week.
For more science, technology, environment and health stories from the BBC, follow us on Facebook and X.
