The mystery origins of Candida auris
 Alamy
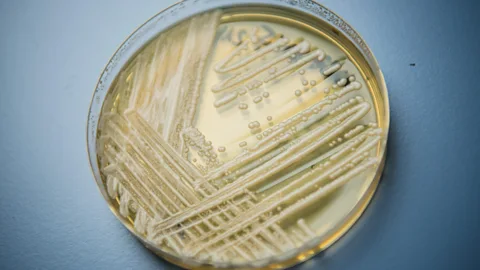
AlamyCandida auris has been found in a wide variety of environments, from oceans to apples.
On a vast palm-fringed beach, bordered by a sapphire-blue sea, a search team was looking for a killer. It was 2021 and the operation was taking place at Corbyn Cove – an impressive swathe of pale golden sand and terracotta beach huts in the Andaman Islands, a remote archipelago in the north-eastern Indian Ocean.
So far, the suspect had appeared in at least 33 countries on three continents, leading to hundreds of deaths. But this was no ordinary manhunt. Firstly, there were no search warrants – only swabs. And secondly, the assailant was a fungus.
Candida auris was first identified in 2009, when a 70-year-old woman turned up at a hospital in Tokyo, Japan, with an ear complaint. Within a few years, hundreds more cases had emerged – and it wasn't long before the yeast took its first victims. Today C. auris has infected tens of thousands of people, including a total of at least 7,413 in the US alone. Earlier this month, the Centers for Disease Control and Prevention (CDC) announced that it is deemed an "urgent" threat for antibiotic resistance – in some areas, the vast majority of cases are resistant to at least one antifungal medication and cases in the country doubled during 2021.
But despite the growing alarm, this new pathogen remains deeply mysterious. No one knows where it originally came from – or why it is spreading so fast.
A rapid conquest
C. auris is a "budding yeast", a microscopic oval-shaped fungus around 2.5-5 micrometres long – roughly the width of a strand of spider silk. The pathogen has specialised in infecting people who are already vulnerable, such as patients with compromised immune systems or pre-existing illnesses. It has swept through hospitals across the globe, lingering in the environment and hopping between patients.
The pathogen is particularly hard to kill – once it has taken up residence on bedding and other surfaces, it can often endure through even the most intensive cleaning. It has been found to remain infectious after drying out on plastic for at least 28 days.
"Even worse, there are few strains of Candida auris which are almost untreatable because they are resistant to the three major classes of drug that we have to treat fungal infections," says Neil Gow, a professor of microbiology at the University of Exeter. "So there are a few really, really worrying strains going around."
Combined with this ability to persist for long periods, C. auris has a fatality rate of between 30 and 72% in infected people – significantly higher than that associated with other antibiotic-resistant microorganisms, which one study estimated at 19%.
An elusive origin
But perhaps the most surprising aspect of C. auris' story so far was uncovered by a team of researchers in 2017. When they analysed the genomes of the fungi found at hospitals in Pakistan, India, South Africa, Venezuela and Japan, they found that while those taken in the same geographic region were closely related, there were three entirely different "strains". Each continent had its own unique version of the pathogen.
This suggests that C. auris has made the leap from the environment into humans at least three times, on three separate occasions, all within the last 14 years. But why? Could there have been a change that has made humans more habitable to the fungus than they were before?
 Alamy
AlamyThe closest known relative of C. auris is C. haemulonii, which has been found in a wide range of environments, from the guts of a fish that inhabits the western Atlantic Ocean to the skin of dolphins and the seas off the coast of Portugal.
Like its cousin, it's thought that C. auris is not restricted to animal hosts, which expands the possibilities for its wild reservoir considerably. But there are some clues. Firstly, C. auris is particularly salt-tolerant, able to withstand concentrations far higher than those that would inhibit the growth of most bacteria. And secondly, it's able to grow well in warm conditions, even thriving at 42C.
Armed with this information, and the knowledge about its closest relative, an international team of scientists decided to survey several environments in the Andaman Islands – a location where they believed the fungal flora would have been less affected by human activity than elsewhere on the planet. They took samples from rocky shores, sandy beaches, marshes, and mangroves around the archipelago and analysed them for evidence of C. auris.
They found evidence of the fungus at two locations – Corbyn Cove Beach and a nearby salt marsh. Could this new pathogen have emerged from the sea?
Unfortunately, just as it looked like the mystery had been solved, another research team made a surprise discovery. C. auris was also found lurking on the surfaces of apples in India – and the strains on the fruit were surprisingly similar to those found in the ocean.
A human cause
The true origins of C. auris remain a total mystery, but both findings back up the leading theories about why the pathogen might have only begun infecting people so recently. The first is climate change.
Ordinarily, fungi are not good at coping with hot conditions. They're much better at infecting animals with cooler body temperatures, such as insects and amphibians. They can totally take over the bodies of the former, leading to a zombie-like state that inspired the video game and TV series The Last of Us. They have also wiped out more than 90 species of amphibian in just 50 years.
This Achilles heel is thought to be one reason that mammals and birds evolved warmer body temperatures, giving us a major advantage in the ongoing battle with these organisms – some experts believe it's partly responsible for the rise of the mammals after dinosaurs went extinct. The factor is so powerful, it's even possible to cure frogs of deadly fungal infections by simply heating them up (don't try this at home). However, these dynamics are changing as the climate warms.
"As they become more adapted to being able to live and thrive at something which approaches body temperature, then of course, it has the chance to grow on a human body," says Gow. If climate change has been heating up environments like tropical beaches, it's plausible that this has allowed C. auris to tolerate the balmy conditions in our bodies.
The other idea is that C. auris owes its emergence to the widespread use of antifungals. Today these drugs and pesticides are liberally applied to suppress microbes from growing on crops, but it's possible that this practice has allowed them to develop resistance. The result is that they can spread much more rapidly among humans than they would have been able to previously – especially in hospitals.
However, despite these discoveries, Gow explains that there have been very few reports of C. auris in a natural environment, "so that's a little bit of an unknown or underexplored area". After all, with the mind-boggling array of different habitats on planet Earth, what are the chances that the few surveys done so far have found the exact locations that it emerged from?
For the moment at least, the research suggests our own species might be to blame for this emerging infectious threat.
--
