Why science still hasn't cracked the artificial heart
 Elizabeth Flores/Alamy
Elizabeth Flores/AlamyFrom tunnelling beneath cities to travelling to the Moon, humanity's engineering achievements are extraordinary. So why has building an artificial heart proved to be more challenging than expected?
Nothing shows more clearly the perfect engineering of the heart than our own failed attempts to imitate it. This history of the total artificial heart is punctuated with both brilliant innovation and continual clinical failure.
In 1962, John F. Kennedy challenged the scientific community to land a man on the moon and return him safely to Earth by the end of the decade. In 1964, cardiovascular surgeon Michael DeBakey persuaded President Lyndon B. Johnson to fund a programme to develop the first functional self-contained artificial heart, launching a race to successfully make one before the moon landing. In 1969 both aims were apparently achieved, with the Texas Heart Institute implanting the first total artificial heart just three months before the launch of Apollo 11. However, while the moon landings have led to the Space Shuttle, Mars Rover, and International Space Station, and (despite a long lull) the newest aims to develop a moon base to bring us to Mars, a reliable off-the-shelf total artificial heart is still just out of reach.
At the outset, the artificial heart was aimed to be a lifetime replacement for the failing organ. This was a high bar to reach, since the first design had an external compressor with an airline through the skin into the patient's body. Compressed air inflated and deflated Dacron pouches or sacs, which collapsed and expanded to displace blood from a surrounding sac. While having the compressor outside the body was useful, since the mechanical parts (which were most susceptible to wear) could be easily replaced, it would make for a bulky piece of equipment to be wheeled about with the patient. It was difficult to see how this could be given to a patient and expect them to live an even partly normal life for many years.
However, the history of the artificial heart is also intertwined with that of the heart transplant. This was again only a hopeful dream in the early 1960s, but by 1967 cardiac surgeon Christian Baarnard in Cape Town performed the first successful transplant. Now, the purpose of these first artificial hearts was changed. They did not need to be suitable for a lifetime; their purpose was to keep the patient alive until a transplant donor could be found. As with many highly experimental therapies, the first case was done on a patient who had run out of options.
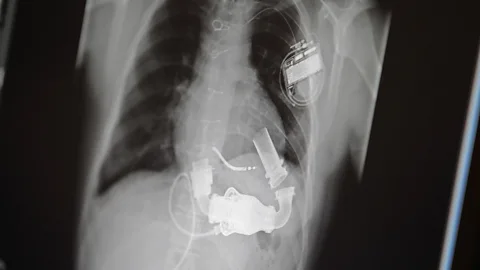
A 47-year-old man was being operated on to repair a huge aneurysm of the left ventricle that had thinned and swollen the heart wall. He was being supported by a heart-lung machine, which bypassed the heart and kept blood flowing through the body. However, he could not be weaned from the machine at the end of the operation as his heart was too weak. He desperately needed a transplant. Denton Cooley, DeBakey's associate, offered him the new experimental total artificial heart and he accepted. The patient was kept stable with the new device for 64 hours until a matching donor heart was found and then transplanted.
This seemed at first a triumph for the total artificial heart, but tragically the patient died 32 hours later from sepsis. Not only that but the device had damaged both the blood and the kidneys, and the walls of the expandable sacs were coated with blood clots. This heralded a series of problems that would continue to thwart the scientists and engineers wrestling with this procedure. Infections and sepsis are a continual challenge to any device where there is a wire that must permanently cross the skin. Devices that move the blood will alter its composition and the foreign surfaces will cause the blood to clot, resulting in strokes and blood breakdown. The first Jarvik heart, one of the next iterations, was implanted in five patients and one lived for 620 days. But two of the patients had severe strokes, and eventually all died of either sepsis or blood problems.
 Brian Nguyen/Alamy
Brian Nguyen/AlamySian E Harding, a recognised authority in cardiac science, is Emeritus Professor of Cardiac Pharmacology in the National Heart and Lung Institute at Imperial College London, where she led the Division of Cardiovascular Sciences and the BHF Centre for Cardiac Regeneration. She is the author of "The Exquisite Machine," from which this article is excerpted.
Heart transplantation also had a shaky start, with Baarnard's first patient dying after only 18 days. The first patient in the UK, whose transplantation was performed at London's National Heart Hospital, survived for only 45 days, and the general success rate remained disappointing. The problem here was not the mechanics of the operation or the initial performance of the new heart. It was the mismatch of the immune system of the recipient to that of the donor heart. Even though the donor heart is matched as closely as possible to the patient with the major tissue types, the immune system must be suppressed to stop the heart being rejected.
You might also like:
Drugs to suppress the immune system were not very sophisticated in the early days, but the development of ciclosporin in the early 1980s produced a revolution in immunosuppression that dramatically improved the success of heart transplantation. Now, it is a victim of its own success, with many more people in need of a transplant than there are donors. Only about 200 transplants are carried out in the UK each year despite more than 750,000 living with heart failure, and similar figures are seen worldwide. To fill this gap, scientists have been genetically modifying pigs to make their hearts compatible with the human immune system so that they can be transplanted to patients without being rejected. This has proved very complex and challenging, but first clinical transplants started in 2022.
The success of heart transplantation, however, had reinvigorated the search for the total artificial heart, with the more achievable goal of keeping the patient alive until a donor is found, or "bridge to transplant" as it is called. For decades, the artificial heart technologies have improved through changes to more biocompatible materials, better valve design, and more efficient handling of blood flow.
Successes have been achieved: one study saw 80% of patients on the artificial hearts surviving for over a year, and some for six years. The longest time a patient was supported to transplant was 1,373 days. But severe infectious complications were still common, and the goal of a complete "destination" therapy for artificial hearts was still a distant dream.
Meanwhile, the urgent need to bridge to transplant had taken the technology in another direction. Rather than replacing the failing heart completely, the idea was to support it by assisting the blood flow. The ventricular assist device, or VAD, took blood out of the ventricle of the heart by a completely different route and pushed it into the aorta at high pressure. This added to the blood being ejected from the heart and thereby magnified the effective cardiac output. It also solved another problem encountered by the engineers of total artificial hearts – how to balance the right and left heart-blood flow.
 Getty Images
Getty ImagesThe amount of blood circulating in the left ventricle/body loop must be very close to that in the right ventricle/lung loop. With 100,000 beats a day, even a teaspoon of difference at each beat would add up to 500 litres (110 gallons) of blood in the wrong place. The heart has evolved complex biological mechanisms to make sure this does not happen, but the engineers were having huge battles to try to do the same with feedback systems. For VADs, either the right (or more usually) the left ventricle can be independently supported, taking this problem away.
Left ventricular assist devices, or LVADs, have produced a revolution in care for end-stage heart failure. More than 15,000 LVADs have now been implanted worldwide, and around a third of patients with end-stage heart failure are now supported on LVADs. The intention is usually to bridge the patients to transplant, but in fact the shortage of donor hearts means that patients can often stay on LVAD support for years. Survival rates of over 50% are seen at seven years, and there are reports of patients living up to 13 years on these devices. LVADs have therefore become by default a therapy in themselves. Again, technology has progressed, with newer LVADs performing better.
A breakthrough idea was to stop imitating the heart, with its pulsing action, and move to constant flow of blood. Rotating paddles (impellers) push the blood along in a continuous motion, creating a smooth unbroken stream. This has the curious side effect of creating a patient without a pulse, which can be disconcerting for the unsuspecting physician as well as producing some unwanted side effects as the body adapts to the new flow.
External battery packs are still an inconvenience and a source of infection, but systems are being developed that transfer energy transcutaneously (across the skin) based on induction (like domestic induction stoves). The LVAD units would still need a small, implanted battery in case of a temporary device failure – and it has been known for external battery packs to be snatched from patients by handbag thieves.
The search for a completely implantable total artificial heart continues. Trying to develop external transcutaneous units to fully power the demands of the heart is the biggest barrier. Specifications for a total artificial heart require it to pump eight litres (14 pints) per minute of blood against a blood pressure of 110mmHg (millimetres of mercury). (The biological power storage molecule adenosine triphosphate [ATP] would be needed in quantities greater than half your body weight per day to power your own heart to do that, if ATP were not continually renewed in cells).
Compressors have been miniaturised to be more portable, but it has been a struggle to make them completely implantable. Here it seems that the VAD technology may hold a solution, dispensing with compressors altogether and using instead the impeller devices, with dual right and left VAD working together.
Solutions seem tantalisingly close, but no one is anticipating an easy ride. The many failures over the years have certainly produced in scientists a humility and awe for the natural engineering of the heart.
* This article originally appeared in The MIT Press Reader, and is republished with permission.
--
