How extinct animals could be brought back from the dead
 Kazuhiro Nogi/Getty Images
Kazuhiro Nogi/Getty ImagesFrom an Australian frog that swallowed its own eggs to woolly mammoths, scientists are getting ever closer to being able to bring long-lost species back from the dead.
Millions of years ago thylacines, also known as Tasmanian tigers, were widespread across Australia. About the size of an American coyote, these dog-like creatures with stripes disappeared from the mainland around 2,000 years ago. They remained in Tasmania until the 1920s, when they were slaughtered by European colonisers who saw them as a threat to livestock.
"It was a human-driven extinction – European settlers came to Australia and brutally obliterated this animal," says Andrew Pask, a geneticist at the University of Melbourne.
Pask is leading a team of scientists who, together with "de-extinction" company Colossal Biosciences, aim to recreate the wolf-like creature and bring it back.
Thanks to recent advances in genetics, namely the advent of gene editing technology Crispr-Cas9, the thylacine is not the only lost species that we could soon see again. How does the science of de-extinction work, and what kinds of ethical questions does it raise?
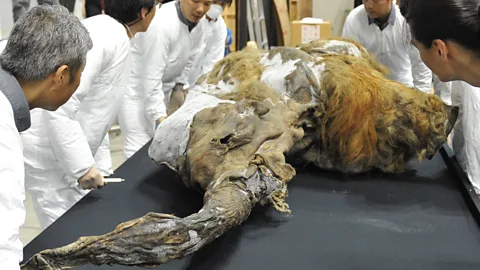
In the case of the thylacine, the first step is sequencing the extinct animal's DNA – the genetic blueprint contained in every single cell of the body. Pask did this in 2017.
"The great thing about the thylacine, is that as it was such an important marsupial every major museum wanted one in their collection, so there are hundreds of samples around the globe, and some are exceptionally preserved," says Pask.
 Sydney Morning Herald/Getty Images
Sydney Morning Herald/Getty Images"Our sample was a baby taken from its mothers' pouch. They shot the mum and immediately dropped the baby into alcohol, which preserves DNA. That was the miracle specimen and the holy grail for us in terms of being able to really build that genome."
Although it's in pretty good condition, the DNA isn't completely whole. Over time, exposure to UV light and the action of bacteria break down DNA into short fragments. The older the sample, the smaller the fragments that are left behind, until eventually there isn't enough left (there's no chance of bringing back a dinosaur, for this reason).
This leaves scientists with the seemingly impossible task of working out how the various bits of DNA fit together – a task comparable to completing an enormous jigsaw without the helpful picture on the front of the box.
Luckily, a small mouse-sized marsupial called a dunnart was able to provide a blueprint.
"We found the closest living relative to the thylacine, which was the dunnart," says Pask.
Dunnarts and thylacines share 95% of their DNA, which is thought to be highly conserved, meaning it hasn't changed much over time.
"We sequenced the dunnart's genome and compared that genetic code to our extinct species, we then overlapped them and found everywhere where it was different," says Pask.
However, knowing an animal's DNA is simply not enough to bring it back. The next stage of the puzzle involves tweaking the genes of the dunnart so that they match the thylacine's. This can be done with Crispr-Cas9, the Nobel Prize-winning genome editing method.
Woolly wonder
Meet the "woolly mouse": a cute rodent with long golden woolly fur.
The genetically-engineered woolly mouse is the brainchild of "de-extinction" company Colossal Biosciences, which wants to resurrect the woolly mammoth using preserved scraps of DNA found frozen in Arctic tundra.
The company also announced in April 2025 that it had bred three young dire wolfs, an animal which went extinct over 10,000 years ago. However, experts have noted that the puppies have important biological differences to dire wolves, disputing the claim that the dire wolf has been brought back from extinction.
With help from Harvard researchers, Colossal Biosciences would like to eventually splice DNA from woolly mammoths into the genomes of Asian elephants to create a "cold-resistant elephant".
"We start with living cells from the dunnart, and we begin to edit all of those changes, so we essentially engineer or turn that dunnart cell into a living thylacine cell with thylacine chromosomes in it," says Pask.
Previously, gene editing wasn't advanced enough to be able to change all of the differing sequences to thylacine DNA in one go. With millions of edits needed, it was assumed that researchers would need to prioritise the most important DNA sequences, yielding an animal genome that wasn't exactly the same as the extinct one. Pask believes this will no longer be necessary.
"All of those technologies are in place, but nobody's done it on this scale before because the DNA-editing technology wasn't good enough or quick enough. But now it's come such a long way that we do have that tech, and we have had significant investment to try and make this work."
Once the researchers have a thylacine cell, they still need to turn it into a developing embryo, and then implant it into a living close-relative's womb. If that sounds easy, then it isn't. "We have a lot of work to do," says Pask.
"We've already made marsupial stem cells which took us about five years. We're now putting those stem cells into embryos to see if we can get them to develop into a whole living animal."
 Auscape/Getty Images
Auscape/Getty ImagesWhat is Crispr?
Crispr-Cas9 was developed by the Nobel-prize winning scientists Emmanuelle Charpentier and Jennifer A Doudna in 2012. The technology harnesses a set of genetic scissors that are part of a defence mechanism used by bacteria. When they encounter a potential viral threat, they copy and paste some of the attacker's DNA into their own genome to create genetic scissors that only slip that exact sequence. Its invention has transformed the speed and cost of edting genes, allowing scientists to accurately delete sections of DNA and create cuts where they can insert new genes.
It isn't just the thylacine that could be brought back this way. Preserved scraps of woolly mammoth DNA found frozen in Arctic tundra mean that this large mammal could return. Most woolly mammoths died out roughly 10,000 years ago.
Scientists at Colossal Laboratories and Bioscience – cofounded by researchers from Harvard University – are using Crispr to splice bits of mammoth DNA into the genome of the Asian elephant, the mammoth's closest living relative. The resulting hybrid, known as a "mammophant'", would be adapted to the cold Siberian tundra, and could help fill an ecological void left by the mammoth when they went extinct. (Watch a film from BBC Reel about how mammoths might be a surprising help in tackling climate change or watch it below.)
There are, however, limitations with the technology, and obstacles that still need to be overcome.
"Many attributes that we have as living animals require several different copies of genes, but it's not easy to tell from looking at a reconstructed genome how many are needed," says Michael Archer, a palaeontologist at the University of New South Wales in Sydney, Australia.
"You keep your fingers crossed that one copy will be sufficient to enable the feature you're looking for, but there's a big suck-it-and-see component to these projects."
However, genome reconstruction is not the only method scientists could use to resurrect extinct animals.
The aurochs, a type of prehistoric cow, is the subject of ancient cave paintings around the globe. It once roamed the plains of Europe and stood as tall as an elephant. It became extinct in the 1600s. Although long gone, auroch genes can still be found in various breeds of cattle around the continent, with descendants in Spain, Portugal, Italy and the Balkans. Geneticists are now "back breeding" these species together to produce offspring closer to the qualities of an auroch.
Another idea is to essentially clone the dead animal by taking the nucleus from an intact cell, and then transferring it into the egg of a close living relative in the hope that an embryo will form.
The caveat is that you need a complete cell to do this, and cells quickly break down after death. An animal like the thylacine that died out almost a hundred years ago simply couldn't be brought back this way.
But it could be an option for recently extinct species.
In 2003, researchers successfully cloned a Pyrenean ibex, a type of goat that went extinct when the last living individual was killed by a falling tree. Sadly, the new-born died of a lung defect shortly after birth.
Archer is currently using a variation of cloning technology to bring back the southern gastric-brooding frog, a species native to Queensland, which became extinct in 1983. The creature had a bizarre method of reproduction, swallowing its fertilised eggs and using its stomach as a sort of womb.
In 2013, he completed the first step – transferring the nucleus from a frozen frog cell into the empty egg of a closely related amphibian. Incredibly the cells started dividing, and an embryo was formed.
"We did it many hundreds of times and it didn't work, and then suddenly one of them did and we saw this hybrid embryo start to divide under the microscope and it was very exciting," says Archer.
After this initial excitement, however, the project stumbled when none of the embryos developed into tadpoles or frogs.
"The frog embryos developed into a ball of cells, which is normal embryonic development, but then they stopped," says Archer.
"Normally the outside layer of cells folds in and you get a two-layered structure which leads to a tadpole, but ours didn't do that."
The same thing happened when the team tried to create an embryo with two living species of frogs, suggesting that it was an aspect of their experimental work that was interfering with the development of the embryo, rather than a problem with the extinct frog's DNA.
"We're working to find out what this obstacle is in living frogs before we can go back to the extinct animal's DNA," says Archer.
 Fine Art/Getty Images
Fine Art/Getty ImagesAre we playing God?
Even if we can bring back extinct animals, there are ethical considerations.
Reintroducing mammoths and thylacines might upset existing ecosystems. Since these animals became extinct, others will have evolved and adapted to fill their place. Will these organisms suffer as a result?
Thanks to climate change, the environments these creatures once lived in may have changed drastically. Some of the plants woolly mammoths fed on are long gone as well. Would mammoths still be able to survive on their own in the wild, and if not, who would look after them? Would they just end up as curiosities in a zoo?
"I don't think we should bring all animals back. I think it should have to fit certain criteria," says Pask.
"For the thylacine it's a recent extinction event, so its habitat in Tasmania still exists, all the food it used to eat still exists, so there's somewhere for them to go and they can thrive again in that environment.
"This animal also played a critical role in the ecosystem. It was an apex predator so it sat right at the top of the food chain. There are no other marsupial apex predators so when it was made extinct it left a massive gap."
Some researchers argue that efforts to bring back long-gone species could detract from conservation efforts to save existing animals and even increase the risk of biodiversity loss, and that people may be less incentivised to stop eating meat and destroying habitats.
But de-extinction technology could be used to save living species on the brink of extinction, especially those with an extremely small gene pool, like the white rhino.
Black headed ferrets are one of North America's most endangered animals – every ferret alive today can trace its ancestry to just seven individuals. Yet researchers at Santiago Zoo in Chile recently took frozen cells from a black footed ferret which died 30 years ago, and used them to create a clone, Elizabeth Ann. Elizabeth's DNA is entirely different, so she can bring a welcome boost of genetic diversity into the population.
"De-extinction tech isn't just about bringing back the thylacine, it's about preventing other animals from becoming extinct," says Pask.
"We have so many bush fires in Australia, and with rising global temperatures we are going to see more adverse weather events in the decades to come. What Australia has been doing is collecting tissues from marsupials in those areas that are most at risk and freezing them. This means that if a bush fire came along, once the vegetation grew back you could repopulate that area with that species."
Archer agrees that the moral rights outweigh any wrongs.
"I think it would be unethical not to do it. I think the ethical issue here was the impropriety of humans making these animals extinct in the first place. It's not about playing God, this is about playing smart human by undoing what we did."
* This article was originally published in 2023 and updated in April 2025 with Colossal Biosciences' announcement that it had bred "dire wolves".
--
